Page 27 - Chalcogenide Glasses for Infrared Optics
P. 27
6 Cha pte r O n e
In general, one may say that good physical properties are the
result of strong chemical bonds formed between atoms of low atomic
mass. The combination of strong bonds (large k) and small atomic
mass (small µ) leads to high vibrational frequencies that fall in the
wavelength region of interest. Oxide materials are not useful in the
longer infrared wavelengths for this reason. Generally speaking, it
will become apparent that infrared optical materials transparent out
to 14 µm do not have good physical properties.
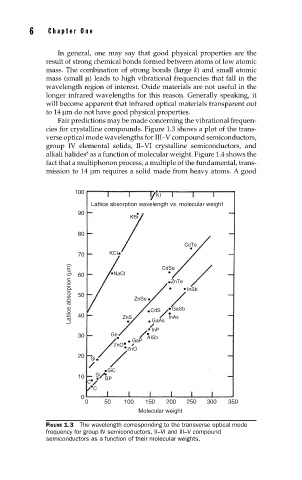
Fair predictions may be made concerning the vibrational frequen-
cies for crystalline compounds. Figure 1.3 shows a plot of the trans-
verse optical mode wavelengths for III–V compound semiconductors,
group IV elemental solids, II–VI crystalline semiconductors, and
8
alkali halides as a function of molecular weight. Figure 1.4 shows the
fact that a multiphonon process, a multiple of the fundamental, trans-
mission to 14 µm requires a solid made from heavy atoms. A good
100
KI
Lattice absorption wavelength vs. molecular weight
90
KBr
80
CdTe
70 KCI CdSe
Lattice absorption (µm) 60 ZnSe CdS ZnTe InSb
NaCl
50
GaSb
40
ZnS
GaAs
InP InAs
30 Ge AISb
GaP
ZnO
ZnO
20
Si
SiC
10 Si BP
C
C
0
0 50 100 150 200 250 300 350
Molecular weight
FIGURE 1.3 The wavelength corresponding to the transverse optical mode
frequency for group IV semiconductors, II–VI and III–V compound
semiconductors as a function of their molecular weights.