Page 43 - Chalcogenide Glasses for Infrared Optics
P. 43
0
0
0
2-8
2
+3
–1
+7
+1
–1
2-7
+6
+4
–2
–2
2-6
+1 +5
+2 –1
+4 –3
+3 –2
–3
+3
+5
2-5
+4
–4
+2
–4
+4
+2
2-4
+3
+3
2-3
+2 2-8-8 2-8-7 2-8-6 2-8-5 2-8-4 2-8-3 +1 +2 +1 +2 0 +4 +3 +2 +3 +5 +6 +5 +4 +2 +3 +3 –1 –2 –3 -8-18-8 -8-18-7 -8-18-6 -8-18-5 -8-18-4 -8-18-3 -8-18-2 -8-18-1 -8-16-2 +1 +4 +3 0 +2 +3 +2 +1 +2 +3 +5 +7 +6 +5 +4 +4 –1 –2 –3 -18-18-8 -18-18-7 -18-1
-8-15-2 -8-16-1 -32-15-2 -32-15-2 Pauling electronegativity
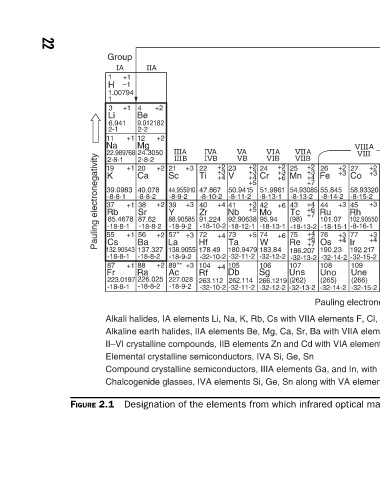
+2 +3 +3 +3 +4 Chalcogenide glasses, IVA elements Si, Ge, Sn along with VA elements P, As, Sb combined with VIA elements S, Se, Te
-8-14-2 -18-15-1 -32-14-2 -32-14-2
+2 +3 +4 +7 +4 +6 +7 +4 +6 +7
-8-13-2 -18-13-2 -32-13-2 -32-13-2 Designation of the elements from which infrared optical materials are formed.
+2 +3 +6 +6 +6 Compound crystalline semiconductors, IIIA elements Ga, and In, with VA elements P, As, Sb
-8-13-1 -18-13-1 -32-12-2 Sg 266.1219 -32-12-2 Alkaline earth halides, IIA elements Be, Mg, Ca, Sr, Ba with VIIA elements F, Cl, Br, I II–VI crystalline compounds, IIB elements Zn and Cd with VIA elements S, Se, Te
+2 +3 +4 +5 +3 +5 +5 Alkali halides, IA elements Li, Na, K, Rb, Cs with VIIA elements F, Cl, Br, I
-8-11-2 -18-12-1 -32-11-2 Db 262.114 -32-11-2
+2 +3 +4 +4 +4 +4
-8-10-2 -18-10-2 -32-10-2 263.112 -32-10-2 Elemental crystalline semiconductors, IVA Si, Ge, Sn
+3 +3 +3 +3
-8-9-2 -18-9-2 -18-9-2 -18-9-2
+2 +2 +2 +2 +2 +2
2-2 2-8-2 -8-8-2 -18-8-2 -18-8-2 -18-8-2
+1 –1 +1 +1 +1 +1 +1 +1 223.0197
2-1 2-8-1 -8-8-1 -18-8-1 -18-8-1 -18-8-1
FIGURE 2.1
Pauling electronegativity
22