Page 45 - Chalcogenide Glasses for Infrared Optics
P. 45
Chalcogenide Glasses 23
A purely covalent bond, such as exists in amorphous selenium
or crystalline silicon, has an even distribution of the bonding elec-
trons between the two atoms, zero percent ionic character. The posi-
tive and negative centers for the atom pair coincide midway between.
A large value of electronegativity indicates a negative element which
tends to attract and hold the bonding electrons closer and away
from the positive element. A low electronegativity indicates a posi-
tive element which tends to furnish the bonding electrons to the
other atom. The positive and negative centers for the atom pair do
27
not coincide. The bond has ionic character. Pauling electronegativity
values for the important elemental families already mentioned are
listed below:
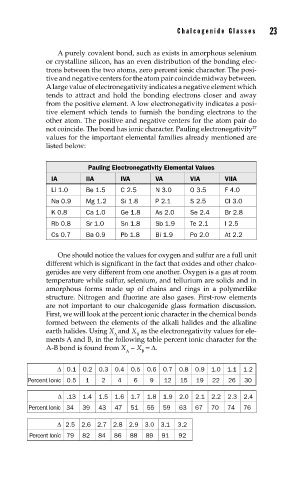
Pauling Electronegativity Elemental Values
IA IIA IVA VA VIA VIIA
Li 1.0 Be 1.5 C 2.5 N 3.0 O 3.5 F 4.0
Na 0.9 Mg 1.2 Si 1.8 P 2.1 S 2.5 Cl 3.0
K 0.8 Ca 1.0 Ge 1.8 As 2.0 Se 2.4 Br 2.8
Rb 0.8 Sr 1.0 Sn 1.8 Sb 1.9 Te 2.1 I 2.5
Cs 0.7 Ba 0.9 Pb 1.8 Bi 1.9 Po 2.0 At 2.2
One should notice the values for oxygen and sulfur are a full unit
different which is significant in the fact that oxides and other chalco-
genides are very different from one another. Oxygen is a gas at room
temperature while sulfur, selenium, and tellurium are solids and in
amorphous forms made up of chains and rings in a polymerlike
structure. Nitrogen and fluorine are also gases. First-row elements
are not important to our chalcogenide glass formation discussion.
First, we will look at the percent ionic character in the chemical bonds
formed between the elements of the alkali halides and the alkaline
earth halides. Using X and X as the electronegativity values for ele-
A B
ments A and B, in the following table percent ionic character for the
A-B bond is found from X − X = ∆.
A B
∆ 0.1 0.2 0.3 0.4 0.5 0.6 0.7 0.8 0.9 1.0 1.1 1.2
Percent Ionic 0.5 1 2 4 6 9 12 15 19 22 26 30
∆ .13 1.4 1.5 1.6 1.7 1.8 1.9 2.0 2.1 2.2 2.3 2.4
Percent Ionic 34 39 43 47 51 55 59 63 67 70 74 76
∆ 2.5 2.6 2.7 2.8 2.9 3.0 3.1 3.2
Percent Ionic 79 82 84 86 88 89 91 92