Page 303 - Challenges in Corrosion Costs Causes Consequences and Control(2015)
P. 303
SHIPS 281
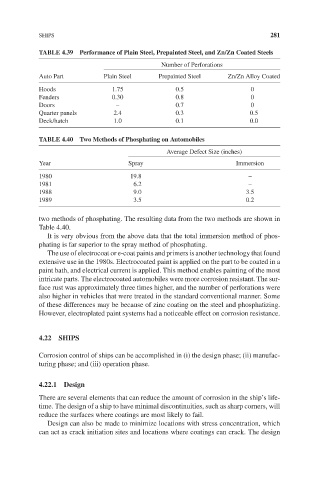
TABLE 4.39 Performance of Plain Steel, Prepainted Steel, and Zn/Zn Coated Steels
Number of Perforations
Auto Part Plain Steel Prepainted Steel Zn/Zn Alloy Coated
Hoods 1.75 0.5 0
Fenders 0.30 0.8 0
Doors – 0.7 0
Quarter panels 2.4 0.3 0.5
Deck/hatch 1.0 0.1 0.0
TABLE 4.40 Two Methods of Phosphating on Automobiles
Average Defect Size (inches)
Year Spray Immersion
1980 19.8 –
1981 6.2 –
1988 9.0 3.5
1989 3.5 0.2
two methods of phosphating. The resulting data from the two methods are shown in
Table 4.40.
It is very obvious from the above data that the total immersion method of phos-
phating is far superior to the spray method of phosphating.
The use of electrocoat or e-coat paints and primers is another technology that found
extensive use in the 1980s. Electrocoated paint is applied on the part to be coated in a
paint bath, and electrical current is applied. This method enables painting of the most
intricate parts. The electrocoated automobiles were more corrosion resistant. The sur-
face rust was approximately three times higher, and the number of perforations were
also higher in vehicles that were treated in the standard conventional manner. Some
of these differences may be because of zinc coating on the steel and phosphatizing.
However, electroplated paint systems had a noticeable effect on corrosion resistance.
4.22 SHIPS
Corrosion control of ships can be accomplished in (i) the design phase; (ii) manufac-
turing phase; and (iii) operation phase.
4.22.1 Design
There are several elements that can reduce the amount of corrosion in the ship’s life-
time. The design of a ship to have minimal discontinuities, such as sharp corners, will
reduce the surfaces where coatings are most likely to fail.
Design can also be made to minimize locations with stress concentration, which
can act as crack initiation sites and locations where coatings can crack. The design