Page 316 - Challenges in Corrosion Costs Causes Consequences and Control(2015)
P. 316
294 CORROSION CONTROL AND PREVENTION
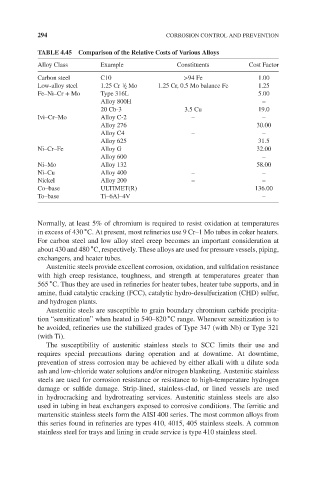
TABLE 4.45 Comparison of the Relative Costs of Various Alloys
Alloy Class Example Constituents Cost Factor
Carbon steel C10 >94 Fe 1.00
1
Low-alloy steel 1.25 Cr / 2 Mo 1.25 Cr, 0.5 Mo balance Fe 1.25
Fe–Ni–Cr + Mo Type 316L 5.00
Alloy 800H –
20 Cb-3 3.5 Cu 19.0
Ivi–Cr–Mo Alloy C-2 – –
Alloy 276 30.00
Alloy C4 – –
Alloy 625 31.5
Ni–Cr–Fe Alloy G 32.00
Alloy 600 –
Ni–Mo Alloy 132 58.00
Ni–Cu Alloy 400 – –
Nickel Alloy 200 – –
Co–base ULTIMET(R) 136.00
To–base Ti–6Al–4V –
Normally, at least 5% of chromium is required to resist oxidation at temperatures
∘
in excess of 430 C. At present, most refineries use 9 Cr–1 Mo tubes in coker heaters.
For carbon steel and low alloy steel creep becomes an important consideration at
∘
about 430 and 480 C, respectively. These alloys are used for pressure vessels, piping,
exchangers, and heater tubes.
Austenitic steels provide excellent corrosion, oxidation, and sulfidation resistance
with high creep resistance, toughness, and strength at temperatures greater than
∘
565 C. Thus they are used in refineries for heater tubes, heater tube supports, and in
amine, fluid catalytic cracking (FCC), catalytic hydro-desulfurization (CHD) sulfur,
and hydrogen plants.
Austenitic steels are susceptible to grain boundary chromium carbide precipita-
∘
tion “sensitization” when heated in 540–820 C range. Whenever sensitization is to
be avoided, refineries use the stabilized grades of Type 347 (with Nb) or Type 321
(with Ti).
The susceptibility of austenitic stainless steels to SCC limits their use and
requires special precautions during operation and at downtime. At downtime,
prevention of stress corrosion may be achieved by either alkali with a dilute soda
ash and low-chloride water solutions and/or nitrogen blanketing. Austenitic stainless
steels are used for corrosion resistance or resistance to high-temperature hydrogen
damage or sulfide damage. Strip-lined, stainless-clad, or lined vessels are used
in hydrocracking and hydrotreating services. Austenitic stainless steels are also
used in tubing in heat exchangers exposed to corrosive conditions. The ferritic and
martensitic stainless steels form the AISI 400 series. The most common alloys from
this series found in refineries are types 410, 4015, 405 stainless steels. A common
stainless steel for trays and lining in crude service is type 410 stainless steel.