Page 319 - Challenges in Corrosion Costs Causes Consequences and Control(2015)
P. 319
CORROSION CONTROL IN THE CHEMICAL, PETROCHEMICAL 297
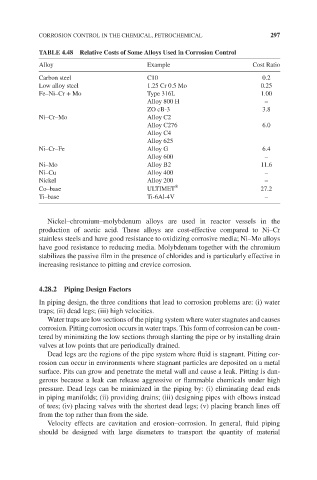
TABLE 4.48 Relative Costs of Some Alloys Used in Corrosion Control
Alloy Example Cost Ratio
Carbon steel C10 0.2
Low alloy steel 1.25 Cr 0.5 Mo 0.25
Fe–Ni–Cr + Mo Type 316L 1.00
Alloy 800 H –
ZO cB-3 3.8
Ni–Cr–Mo Alloy C2
Alloy C276 6.0
Alloy C4
Alloy 625
Ni–Cr–Fe Alloy G 6.4
Alloy 600 –
Ni–Mo Alloy B2 11.6
Ni–Cu Alloy 400 –
Nickel Alloy 200 –
®
Co–base ULTIMET 27.2
Ti–base Ti-6Al-4V –
Nickel–chromium–molybdenum alloys are used in reactor vessels in the
production of acetic acid. These alloys are cost-effective compared to Ni–Cr
stainless steels and have good resistance to oxidizing corrosive media; Ni–Mo alloys
have good resistance to reducing media. Molybdenum together with the chromium
stabilizes the passive film in the presence of chlorides and is particularly effective in
increasing resistance to pitting and crevice corrosion.
4.28.2 Piping Design Factors
In piping design, the three conditions that lead to corrosion problems are: (i) water
traps; (ii) dead legs; (iii) high velocities.
Water traps are low sections of the piping system where water stagnates and causes
corrosion. Pitting corrosion occurs in water traps. This form of corrosion can be coun-
tered by minimizing the low sections through slanting the pipe or by installing drain
valves at low points that are periodically drained.
Dead legs are the regions of the pipe system where fluid is stagnant. Pitting cor-
rosion can occur in environments where stagnant particles are deposited on a metal
surface. Pits can grow and penetrate the metal wall and cause a leak. Pitting is dan-
gerous because a leak can release aggressive or flammable chemicals under high
pressure. Dead legs can be minimized in the piping by: (i) eliminating dead ends
in piping manifolds; (ii) providing drains; (iii) designing pipes with elbows instead
of tees; (iv) placing valves with the shortest dead legs; (v) placing branch lines off
from the top rather than from the side.
Velocity effects are cavitation and erosion–corrosion. In general, fluid piping
should be designed with large diameters to transport the quantity of material