Page 368 - Challenges in Corrosion Costs Causes Consequences and Control(2015)
P. 368
346 CONSEQUENCES OF CORROSION
The high amounts of Al and Zn reported may be because of the use of galvanized
pipes and a sacrificial anode cathodic protection system in the hot water tank.
5.3.7.4.7 Cathodic Protection of Steel in Concrete More than 5 billion dollars are
spent annually in repairs to concrete structures such as buildings, bridges, parking
garages, and other structures. Carbon dioxide enters the concrete and reacts with the
lime, forming carbonic acid, and lowers the pH of the medium. Further, the chloride
in deicing salts along with oxygen and water creates an aggressive corrosive envi-
ronment. This results in an electrochemical reaction leading to delamination. The
rebar corrodes, and the resulting rust is voluminous, leading to cracking, spalling,
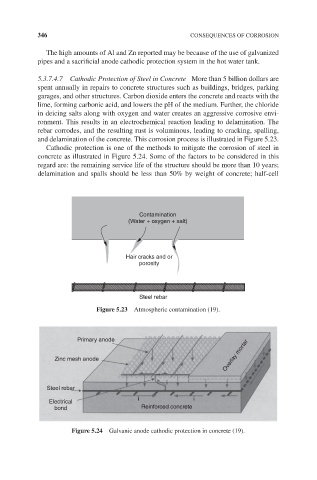
and delamination of the concrete. This corrosion process is illustrated in Figure 5.23.
Cathodic protection is one of the methods to mitigate the corrosion of steel in
concrete as illustrated in Figure 5.24. Some of the factors to be considered in this
regard are: the remaining service life of the structure should be more than 10 years;
delamination and spalls should be less than 50% by weight of concrete; half-cell
Contamination
(Water + oxygen + salt)
Hair cracks and or
porosity
Steel rebar
Figure 5.23 Atmospheric contamination (19).
Primary anode
Overlay mortar
Zinc mesh anode
i
Steel rebar
i i
Electrical
bond Reinforced concrete
Figure 5.24 Galvanic anode cathodic protection in concrete (19).