Page 371 - Challenges in Corrosion Costs Causes Consequences and Control(2015)
P. 371
CORROSION DAMAGE, DEFECTS, AND FAILURES 349
Figure 5.28 Aluminum corrosion products on interior surface of dress cap. (Figure originally
published in Reference 26. Reproduced with permission of the Canadian Institute of Mining,
Metallurgy and Petroleum. www.cim.org.)
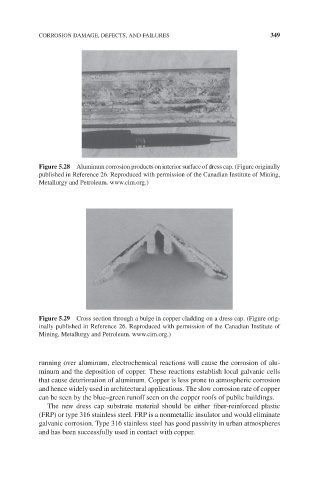
Figure 5.29 Cross section through a bulge in copper cladding on a dress cap. (Figure orig-
inally published in Reference 26. Reproduced with permission of the Canadian Institute of
Mining, Metallurgy and Petroleum. www.cim.org.)
running over aluminum, electrochemical reactions will cause the corrosion of alu-
minum and the deposition of copper. These reactions establish local galvanic cells
that cause deterioration of aluminum. Copper is less prone to atmospheric corrosion
and hence widely used in architectural applications. The slow corrosion rate of copper
can be seen by the blue–green runoff seen on the copper roofs of public buildings.
The new dress cap substrate material should be either fiber-reinforced plastic
(FRP) or type 316 stainless steel. FRP is a nonmetallic insulator and would eliminate
galvanic corrosion. Type 316 stainless steel has good passivity in urban atmospheres
and has been successfully used in contact with copper.