Page 77 - Characterization and Properties of Petroleum Fractions
P. 77
T1: IML
QC: —/—
P2: —/—
P1: KVU/—
16:6
August 16, 2007
AT029-Manual-v7.cls
AT029-Manual
AT029-02
2. CHARACTERIZATION AND PROPERTIES OF PURE HYDROCARBONS 57
API Gravity
Kinematic Viscosity at 100°F , cSt Kinematic Viscosity at 210°F , cSt Molecular Weight
--`,```,`,``````,`,````,```,,-`-`,,`,,`,`,,`---
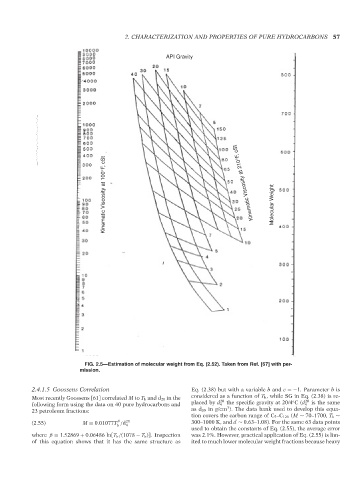
FIG. 2.5—Estimation of molecular weight from Eq. (2.52). Taken from Ref. [67] with per-
mission.
2.4.1.5 Goossens Correlation Eq. (2.38) but with a variable b and c =−1. Parameter b is
Most recently Goossens [61] correlated M to T b and d 20 in the considered as a function of T b , while SG in Eq. (2.38) is re-
20
20
◦
following form using the data on 40 pure hydrocarbons and placed by d 4 the specific gravity at 20/4 C(d 4 is the same
3
23 petroleum fractions: as d 20 in g/cm ). The data bank used to develop this equa-
tion covers the carbon range of C 5 –C 120 (M ∼ 70–1700, T b ∼
β
(2.55) M = 0.01077T /d 20 300–1000 K, and d ∼ 0.63–1.08). For the same 63 data points
b 4
used to obtain the constants of Eq. (2.55), the average error
where β = 1.52869 + 0.06486 ln[T b /(1078 − T b )]. Inspection was 2.1%. However, practical application of Eq. (2.55) is lim-
of this equation shows that it has the same structure as ited to much lower molecular weight fractions because heavy
Copyright ASTM International
Provided by IHS Markit under license with ASTM Licensee=International Dealers Demo/2222333001, User=Anggiansah, Erick
No reproduction or networking permitted without license from IHS Not for Resale, 08/26/2021 21:56:35 MDT