Page 137 - Chemical and process design handbook
P. 137
Speight_Part II_B 11/7/01 3:11 PM Page 2.78
2.78 MANUFACTURE OF CHEMICALS
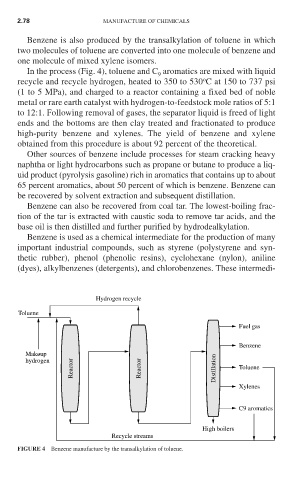
Benzene is also produced by the transalkylation of toluene in which
two molecules of toluene are converted into one molecule of benzene and
one molecule of mixed xylene isomers.
In the process (Fig. 4), toluene and C aromatics are mixed with liquid
9
o
recycle and recycle hydrogen, heated to 350 to 530 C at 150 to 737 psi
(1 to 5 MPa), and charged to a reactor containing a fixed bed of noble
metal or rare earth catalyst with hydrogen-to-feedstock mole ratios of 5:1
to 12:1. Following removal of gases, the separator liquid is freed of light
ends and the bottoms are then clay treated and fractionated to produce
high-purity benzene and xylenes. The yield of benzene and xylene
obtained from this procedure is about 92 percent of the theoretical.
Other sources of benzene include processes for steam cracking heavy
naphtha or light hydrocarbons such as propane or butane to produce a liq-
uid product (pyrolysis gasoline) rich in aromatics that contains up to about
65 percent aromatics, about 50 percent of which is benzene. Benzene can
be recovered by solvent extraction and subsequent distillation.
Benzene can also be recovered from coal tar. The lowest-boiling frac-
tion of the tar is extracted with caustic soda to remove tar acids, and the
base oil is then distilled and further purified by hydrodealkylation.
Benzene is used as a chemical intermediate for the production of many
important industrial compounds, such as styrene (polystyrene and syn-
thetic rubber), phenol (phenolic resins), cyclohexane (nylon), aniline
(dyes), alkylbenzenes (detergents), and chlorobenzenes. These intermedi-
Hydrogen recycle
Toluene
Fuel gas
Benzene
Make-up
hydrogen
Reactor Reactor Distillation Toluene
Xylenes
C9 aromatics
High boilers
Recycle streams
FIGURE 4 Benzene manufacture by the transalkylation of toluene.