Page 190 - Chemical and process design handbook
P. 190
Speight_Part II_C 11/7/01 3:08 PM Page 2.131
CALCIUM OXIDE
Calcium oxide (CaO, lime, quicklime, unslaked lime) is differentiated
from calcium hydroxide [Ca(OH) , slaked lime, hydrated lime] and lime-
2
stone (CaCO , calcite, calcium carbonate, marble chips, chalk) by formula
3
and by behavior. A saturated solution of calcium oxide in water is called
limewater and a suspension in water is called milk of lime.
Lime is manufactured by calcining or heating limestone in a kiln.
CaCO → CaO +CO
3 2
Temperatures used in converting limestone into lime are on the order of
o
1200 to 1300 C.
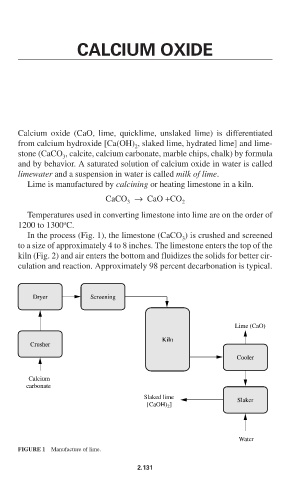
In the process (Fig. 1), the limestone (CaCO ) is crushed and screened
3
to a size of approximately 4 to 8 inches. The limestone enters the top of the
kiln (Fig. 2) and air enters the bottom and fluidizes the solids for better cir-
culation and reaction. Approximately 98 percent decarbonation is typical.
Dryer Screening
Lime (CaO)
Kiln
Crusher
Cooler
Calcium
carbonate
Slaked lime Slaker
[CaOH) ]
2
Water
FIGURE 1 Manufacture of lime.
2.131