Page 191 - Chemical and process design handbook
P. 191
Speight_Part II_C 11/7/01 3:08 PM Page 2.132
2.132 MANUFACTURE OF CHEMICALS
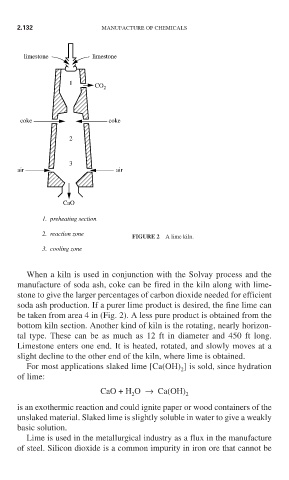
limestone limestone
1
CO
2
coke coke
2
3
air air
CaO
1. preheating section
2. reaction zone
FIGURE 2 A lime kiln.
3. cooling zone
When a kiln is used in conjunction with the Solvay process and the
manufacture of soda ash, coke can be fired in the kiln along with lime-
stone to give the larger percentages of carbon dioxide needed for efficient
soda ash production. If a purer lime product is desired, the fine lime can
be taken from area 4 in (Fig. 2). A less pure product is obtained from the
bottom kiln section. Another kind of kiln is the rotating, nearly horizon-
tal type. These can be as much as 12 ft in diameter and 450 ft long.
Limestone enters one end. It is heated, rotated, and slowly moves at a
slight decline to the other end of the kiln, where lime is obtained.
For most applications slaked lime [Ca(OH) ] is sold, since hydration
2
of lime:
CaO + H O → Ca(OH)
2 2
is an exothermic reaction and could ignite paper or wood containers of the
unslaked material. Slaked lime is slightly soluble in water to give a weakly
basic solution.
Lime is used in the metallurgical industry as a flux in the manufacture
of steel. Silicon dioxide is a common impurity in iron ore that cannot be