Page 204 - Chemical and process design handbook
P. 204
Speight_Part II_C 11/7/01 3:08 PM Page 2.144
2.144 MANUFACTURE OF CHEMICALS
varying purity and crystalline size and perfection. For import purposes,
natural graphite is classified as crystalline and amorphous The latter is not
.
truly amorphous but has an imperfect lamellar microcrystalline structure.
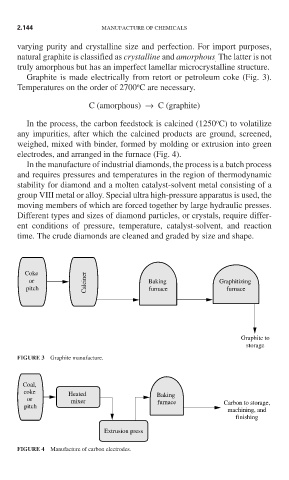
Graphite is made electrically from retort or petroleum coke (Fig. 3).
o
Temperatures on the order of 2700 C are necessary.
C (amorphous) → C (graphite)
o
In the process, the carbon feedstock is calcined (1250 C) to volatilize
any impurities, after which the calcined products are ground, screened,
weighed, mixed with binder, formed by molding or extrusion into green
electrodes, and arranged in the furnace (Fig. 4).
In the manufacture of industrial diamonds, the process is a batch process
and requires pressures and temperatures in the region of thermodynamic
stability for diamond and a molten catalyst-solvent metal consisting of a
group VIII metal or alloy. Special ultra high-pressure apparatus is used, the
moving members of which are forced together by large hydraulic presses.
Different types and sizes of diamond particles, or crystals, require differ-
ent conditions of pressure, temperature, catalyst-solvent, and reaction
time. The crude diamonds are cleaned and graded by size and shape.
Coke
or Calciner Baking Graphitizing
pitch furnace furnace
Graphite to
storage
FIGURE 3 Graphite manufacture.
Coal,
coke Heated Baking
or
mixer furnace Carbon to storage,
pitch
machining, and
finishing
Extrusion press
FIGURE 4 Manufacture of carbon electrodes.