Page 99 - Chemical and process design handbook
P. 99
Speight_Part II_A 11/7/01 3:16 PM Page 2.40
2.40 MANUFACTURE OF CHEMICALS
In this process, the fine powder of lithium phosphate used as catalyst is
o
dispersed, and propylene oxide is fed at 300 C to the reactor, and the prod-
uct, allyl alcohol, together with unreacted propylene oxide is removed by
distillation. By-products such as acetone and propionaldehyde, which are
isomers of propylene oxide, are formed, but the conversion of propylene
oxide is 40 percent and the selectivity to allyl alcohol reaches more than 90
percent. Allyl alcohol obtained by this process may contain small amounts
(<1%) of propanol.
The fourth process for the production of allyl alcohol was developed
partly for the purpose of producing epichlorohydrin via allyl alcohol as the
intermediate, using a palladium catalyst.
CH CH=CH + CH COOH + O → CH =CHOCOCH
3 2 3 2 2 3
CH =CHOCOCH + H O → CH =CHCH OH + CH CO H
2 3 2 2 2 3 2
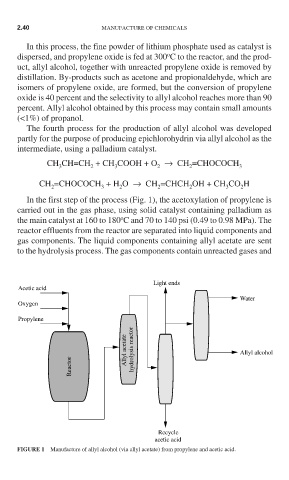
In the first step of the process (Fig. 1), the acetoxylation of propylene is
carried out in the gas phase, using solid catalyst containing palladium as
o
the main catalyst at 160 to 180 C and 70 to 140 psi (0.49 to 0.98 MPa). The
reactor effluents from the reactor are separated into liquid components and
gas components. The liquid components containing allyl acetate are sent
to the hydrolysis process. The gas components contain unreacted gases and
Light ends
Acetic acid
Water
Oxygen
Propylene
Allyl acetate hydrolysis reactor Allyl alcohol
Reactor
Recycle
acetic acid
FIGURE 1 Manufacture of allyl alcohol (via allyl acetate) from propylene and acetic acid.