Page 103 - Chemical and process design handbook
P. 103
Speight_Part II_A 11/7/01 3:16 PM Page 2.44
ALUMINUM
o
o
Aluminum (melting point: 660 C, boiling point: 2494 C) is the most abun-
dant metal in the world and makes up 7 to 10% by weight of the earth’s crust.
Aluminum is manufactured by the electrolytic reduction of pure alu-
mina (Al O ) in a bath of fused cryolite (Na AlF ). It is not possible to
2 3 3 6
reduce alumina with carbon because aluminum carbide (A1 C ) is formed
4 3
and a back-reaction between aluminum vapor and carbon dioxide in the
condenser quickly reforms the original aluminum oxide again.
The electrolytic cells are large containers (usually steel), and each is a
cathode compartment lined with either a mixture of pitch and anthracite
coal or coke baked in place by the passage of electric current or prebaked
cathode blocks cemented together.
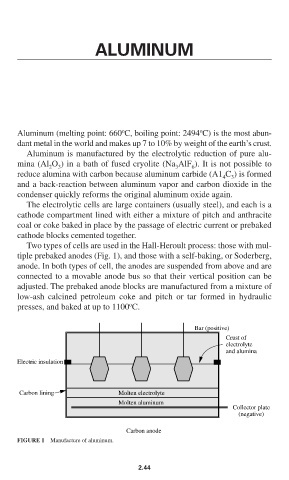
Two types of cells are used in the Hall-Heroult process: those with mul-
tiple prebaked anodes (Fig. 1), and those with a self-baking, or Soderberg,
anode. In both types of cell, the anodes are suspended from above and are
connected to a movable anode bus so that their vertical position can be
adjusted. The prebaked anode blocks are manufactured from a mixture of
low-ash calcined petroleum coke and pitch or tar formed in hydraulic
o
presses, and baked at up to 1100 C.
Bar (positive)
Crust of
electrolyte
and alumina
Electric insulation
Carbon lining Molten electrolyte
Molten aluminum
Collector plate
(negative)
Carbon anode
FIGURE 1 Manufacture of aluminum.
2.44