Page 106 - Chemical and process design handbook
P. 106
Speight_Part II_A 11/7/01 3:16 PM Page 2.47
AMITRIPTYLINE
Amitriptyline hydrochloride and imipramine hydrochloride are similar in
structure, with the exception of the nitrogen in the center ring, and belong
to the family of phenothiazine compounds. Finally, the two-carbon bridge
linking the aromatic rings may be ethyl (–CH CH –) or ethylene
2 2
(–CH=CH–).
These compounds are central nervous system stimulants or antidepres-
sants although such activity is usually restricted to compounds having a
two- or three-carbon side chain and methyl-substituted or unsubstituted
amino groups in the side chain although derivatives with substituents on
the aromatic ring may have pharmacological activity.
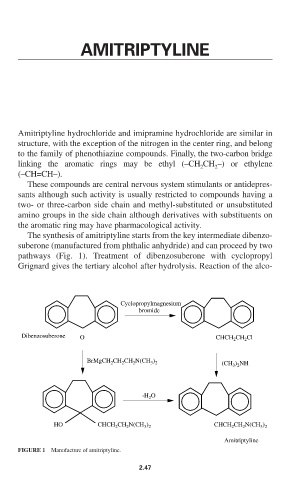
The synthesis of amitriptyline starts from the key intermediate dibenzo-
suberone (manufactured from phthalic anhydride) and can proceed by two
pathways (Fig. 1). Treatment of dibenzosuberone with cyclopropyl
Grignard gives the tertiary alcohol after hydrolysis. Reaction of the alco-
Cyclopropylmagnesium
bromide
Dibenzosuberone O CHCH CH Cl
2
2
BrMgCH CH CH N(CH ) (CH 3 2
2
3 2
2
) NH
2
-H O
2
HO CHCH CH N(CH ) CHCH CH N(CH )
2
3 2
2
2
3 2
2
Amitriptyline
FIGURE 1 Manufacture of amitriptyline.
2.47