Page 170 - Chemical engineering design
P. 170
147
FLOW-SHEETING
In the production of dichloroethane (EDC) by oxyhydrochlorination of ethylene, the
products from the reaction are quenched by direct contact with dilute HCl in a quench
tower. The gaseous stream from this quench tower is fed to a condenser and the uncon-
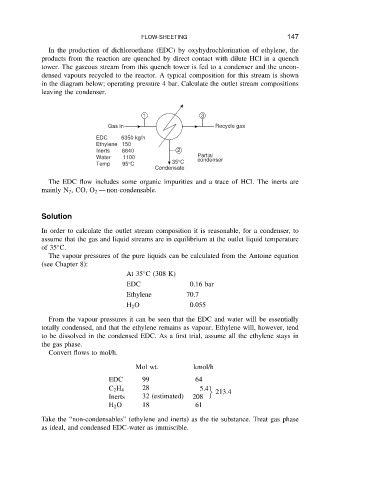
densed vapours recycled to the reactor. A typical composition for this stream is shown
in the diagram below; operating pressure 4 bar. Calculate the outlet stream compositions
leaving the condenser.
1 3
Gas in Recycle gas
EDC 6350 kg/h
Ethylene 150
Inerts 6640 2
Water 1100 Partial
condenser
Temp 95°C 35°C
Condensate
The EDC flow includes some organic impurities and a trace of HCl. The inerts are
non-condensable.
mainly N 2 ,CO, O 2
Solution
In order to calculate the outlet stream composition it is reasonable, for a condenser, to
assume that the gas and liquid streams are in equilibrium at the outlet liquid temperature
Ž
of 35 C.
The vapour pressures of the pure liquids can be calculated from the Antoine equation
(see Chapter 8):
Ž
At 35 C (308 K)
EDC 0.16 bar
Ethylene 70.7
H 2 O 0.055
From the vapour pressures it can be seen that the EDC and water will be essentially
totally condensed, and that the ethylene remains as vapour. Ethylene will, however, tend
to be dissolved in the condensed EDC. As a first trial, assume all the ethylene stays in
the gas phase.
Convert flows to mol/h.
Mol wt. kmol/h
EDC 99 64
28 5.4
C 2 H 4 213.4
Inerts 32 (estimated) 208
H 2 O 18 61
Take the “non-condensables” (ethylene and inerts) as the tie substance. Treat gas phase
as ideal, and condensed EDC-water as immiscible.