Page 172 - Chemical engineering design
P. 172
149
FLOW-SHEETING
This is little different from calculated value and shows that initial assumption that
no ethylene was condensed or dissolved was reasonable; so report ethylene in liquid
as “trace”.
Material balance Flows (kg/h)
Stream no.: 1 2 3
Title Condenser feed Condensate Recycle gas
EDC 6350 5459 891
H 2 O 1100 1044 56
Ethylene 150 Trace 150
Inerts 6640 6640
Total 14,240 6503 7737
Ž
Temp. C 95 35 35
Pressure bar: 4 4 4
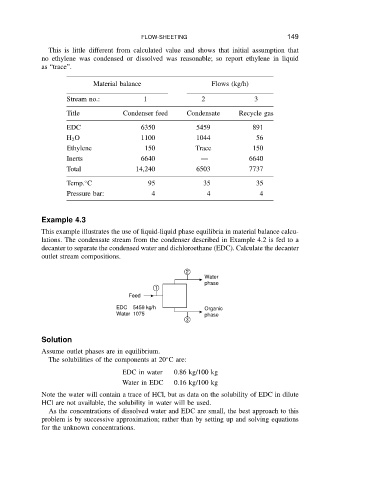
Example 4.3
This example illustrates the use of liquid-liquid phase equilibria in material balance calcu-
lations. The condensate stream from the condenser described in Example 4.2 is fed to a
decanter to separate the condensed water and dichloroethane (EDC). Calculate the decanter
outlet stream compositions.
2
Water
phase
1
Feed
EDC 5459 kg/h Organic
Water 1075 phase
3
Solution
Assume outlet phases are in equilibrium.
Ž
The solubilities of the components at 20 Care:
EDC in water 0.86 kg/100 kg
Water in EDC 0.16 kg/100 kg
Note the water will contain a trace of HCl, but as data on the solubility of EDC in dilute
HCl are not available, the solubility in water will be used.
As the concentrations of dissolved water and EDC are small, the best approach to this
problem is by successive approximation; rather than by setting up and solving equations
for the unknown concentrations.