Page 105 - Chemical equilibria Volume 4
P. 105
Molecular Chemical Equilibria 81
Thus, generally speaking, a metal is capable of reducing an oxide if its
Ellingham line is situated below that of the oxide.
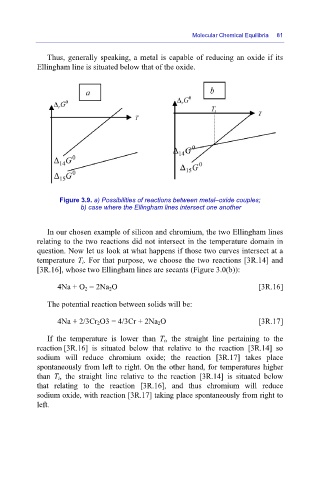
Figure 3.9. a) Possibilities of reactions between metal–oxide couples;
b) case where the Ellingham lines intersect one another
In our chosen example of silicon and chromium, the two Ellingham lines
relating to the two reactions did not intersect in the temperature domain in
question. Now let us look at what happens if those two curves intersect at a
temperature T i. For that purpose, we choose the two reactions [3R.14] and
[3R.16], whose two Ellingham lines are secants (Figure 3.0(b)):
4Na + O 2 = 2Na 2O [3R.16]
The potential reaction between solids will be:
4Na + 2/3Cr 2O3 = 4/3Cr + 2Na 2O [3R.17]
If the temperature is lower than T i, the straight line pertaining to the
reaction [3R.16] is situated below that relative to the reaction [3R.14] so
sodium will reduce chromium oxide; the reaction [3R.17] takes place
spontaneously from left to right. On the other hand, for temperatures higher
than T i, the straight line relative to the reaction [3R.14] is situated below
that relating to the reaction [3R.16], and thus chromium will reduce
sodium oxide, with reaction [3R.17] taking place spontaneously from right to
left.