Page 109 - Chemical equilibria Volume 4
P. 109
Molecular Chemical Equilibria 85
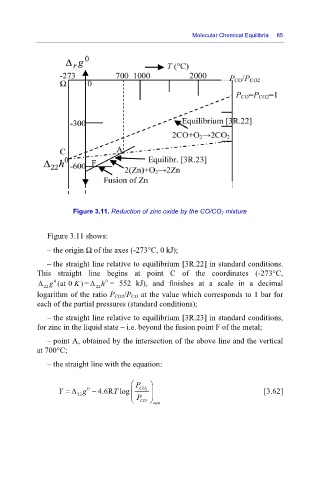
Figure 3.11. Reduction of zinc oxide by the CO/CO 2 mixture
Figure 3.11 shows:
– the origin Ω of the axes (-273°C, 0 kJ);
– the straight line relative to equilibrium [3R.22] in standard conditions.
This straight line begins at point C of the coordinates (-273°C,
0
)
Δ g 0 (at 0 K = Δ h = 552 kJ), and finishes at a scale in a decimal
22 22
logarithm of the ratio P CO2/P CO at the value which corresponds to 1 bar for
each of the partial pressures (standard conditions);
– the straight line relative to equilibrium [3R.23] in standard conditions,
for zinc in the liquid state – i.e. beyond the fusion point F of the metal;
– point A, obtained by the intersection of the above line and the vertical
at 700°C;
– the straight line with the equation:
⎛ P ⎞
Y = Δ g − 4.6R log⎜ T ⎜ CO 2 ⎟ ⎟ [3.62]
0
22
⎝ P CO ⎠ equ