Page 119 - Chemical equilibria Volume 4
P. 119
Molecular Chemical Equilibria 95
3.5.3. Iso-Q curves in perfect solutions
To find the general equation for iso-parametric curves, or “iso-Q” curves, we
feed the values of x 1, x 2 and x 3 as functions of x and y (expressions [3.75]) back
into the application of the law of mass action [3.77]. For the equation of iso-Q
curves, we find:
y 3 β .2 ( 1 β − 2 β ) = Q [3.89]
1 β
y
( x 3 − y ) ( 2 − − x ) 3 2 β x
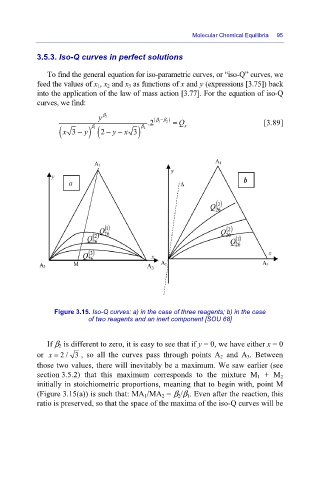
Figure 3.15. Iso-Q curves: a) in the case of three reagents; b) in the case
of two reagents and an inert component [SOU 68]
If β 2 is different to zero, it is easy to see that if y = 0, we have either x = 0
or x = 2/ 3 , so all the curves pass through points A 2 and A 3. Between
those two values, there will inevitably be a maximum. We saw earlier (see
section 3.5.2) that this maximum corresponds to the mixture M 1 + M 2
initially in stoichiometric proportions, meaning that to begin with, point M
(Figure 3.15(a)) is such that: MA 1/MA 2 = β 2/β 1. Even after the reaction, this
ratio is preserved, so that the space of the maxima of the iso-Q curves will be