Page 361 - Chemical process engineering design and economics
P. 361
340 Chapter 6
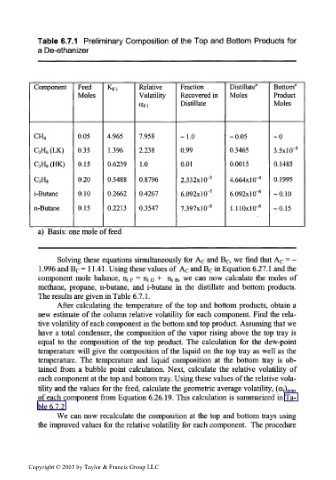
Table 6.7.1 Preliminary Composition of the Top and Bottom Products for
a De-ethanizer
Component Feed K Fi Relative Fraction Distillate" Bottom a
Moles Volatility Recovered in Moles Product
Distillate Moles
«Fi
CH 4 0.05 4.965 7.958 -1.0 -0.05 -0
C 2H 6(LK) 0.35 1.396 2.238 0.99 0.3465 3.5xlO~ 3
C 3H 6(HK) 0.15 0.6239 1.0 0.01 0.0015 0.1485
C 3H 8 0.20 0.5488 0.8796 2.332xlO" 3 4.664x10"" 0.1995
i-Butane 0.10 0.2662 0.4267 6.092xlO" 7 6.092xlO~ 8 -0.10
n-Butane 0.15 0.2213 0.3547 7.397xlO~ 8 1.110xlO~ 8 -0.15
a) Basis: one mole of feed
Solving these equations simultaneously for A c and B c, we find that A c = -
1.996 and B c = 11.41. Using these values of A c and B c in Equation 6.27.1 and the
component mole balance, n ; F = n ; D + u iB, we can now calculate the moles of
methane, propane, n-butane, and i-butane in the distillate and bottom products.
The results are given in Table 6.7.1.
After calculating the temperature of the top and bottom products, obtain a
new estimate of the column relative volatility for each component. Find the rela-
tive volatility of each component in the bottom and top product. Assuming that we
have a total condenser, the composition of the vapor rising above the top tray is
equal to the composition of the top product. The calculation for the dew-point
temperature will give the composition of the liquid on the top tray as well as the
temperature. The temperature and liquid composition at the bottom tray is ob-
tained from a bubble point calculation. Next, calculate the relative volatility of
each component at the top and bottom tray. Using these values of the relative vola-
tility and the values for the feed, calculate the geometric average volatility, (ocj) avg,
of each component from Equation 6.26.19. This calculation is summarized in Ta-
ble 6.7.2
We can now recalculate the composition at the top and bottom trays using
the improved values for the relative volatility for each component. The procedure
Copyright © 2003 by Taylor & Francis Group LLC