Page 112 - Chiral Separation Techniques
P. 112
88 3 Combinatorial Approaches to Recognition of Chirality: Preparation …
In the next step, each single amino acid was coupled separately with the 12
amines resulting in three new CSPs (CSP 15–CSP 17) each containing mixed li-
gands. Once packed into HPLC columns these CSPs were evaluated. The highest
selectivity factor of 13.7 in the first deconvoluted series of columns was found for
the proline-based CSP 17 while the α-values of the two other columns were close to
5. Further deconvolution of the proline-based column was carried out by splitting the
12 members of the amine building blocks 26–37 in two subgroups to afford a third
set of columns (CSP 18 and CSP 19). Thus, the first proline-based sublibrary col-
umn (CSP 18) was prepared using 6 amines with smaller aromatic substituents
(amines 26–31), and the second column (CSP 19) with amines having larger sub-
stituents (32–37). These columns exhibited selectivity factors of 13.6 and 7.3,
respectively. In the next step, the 6 amine-based groups present in the more selective
column CSP 18 were divided again into 2 groups (26–28 and 29–31), containing 3
amines with mainly meta-substituted aromatic amines and another 3 with para-sub-
stituted amines. The respective columns CSP 20 and CSP 21 exhibited rather high
α-values of 17.4 and 14.9 for racemic (3,5-dinitrobenzoyl)leucine diallylamide and
indicated that both groups involved at least one selector with a very high selectivity.
This was not surprising for CSP 20, since we have previously demonstrated that the
selector substituted with 3,5-dimethylaniline (27) that was intentionally used in our
experimental design, was quite powerful [8]. Since the performance of these two
columns was similar, we decided to further deconvolute CSP 21 as it involved an
entirely new set of selectors. Three columns CSP 22–CSP 24 packed with beads con-
taining only individual selectors were prepared with the amines 29, 30, and 31,
respectively. Although all these columns exhibited rather high selectivities, an α-
value of 23.1 was achieved with CSP 22 featuring 5-aminoindane 29 as a part of the
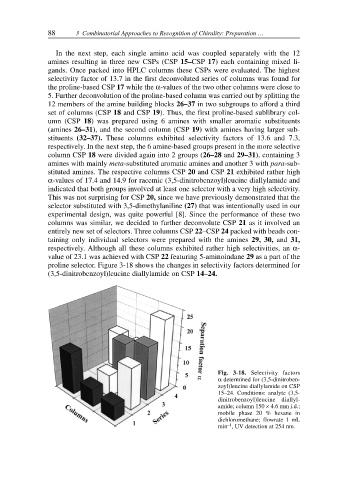
proline selector. Figure 3-18 shows the changes in selectivity factors determined for
(3,5-dinitrobenzoyl)leucine diallylamide on CSP 14–24.
Fig. 3-18. Selectivity factors
α determined for (3,5-dinitroben-
zoyl)leucine diallylamide on CSP
15–24. Conditions: analyte (3,5-
dinitrobenzoyl)leucine diallyl-
amide; column 150 × 4.6 mm i.d.;
mobile phase 20 % hexane in
dichloromethane; flowrate 1 mL
–1
min , UV detection at 254 nm.