Page 107 - Chiral Separation Techniques
P. 107
3.7 Combinatorial Libraries of Selectors for HPLC 83
CSP 12 afforded good separations for a variety of racemic α-amino acid deriva-
tives. Figure 3-14B shows an example of a typical separation of 3,5-dinitrobenz-
amidoalanine-N,N-diethylamide enantiomers with a separation factor α = 7.7. Even
though CSP 12 was designed for the separation of derivatized amino acids, other
classes of compounds such as dihydropyrimidines and profens could also be
resolved. In addition, this phase could be utilized under reversed-phase separation
conditions. Despite the suppression of the hydrogen bonding interactions between
the CSP and analyte, rather good enantioselectivities (α = 3.5) were obtained even
in a mobile phase consisting of 50 % water. This confirmed that CSP 12 was a ver-
satile phase capable of enantioseparations in either reversed-phase or normal-phase
mode.
The reciprocal strategy is best suited for typical situations encountered in the
industry that require the preparation of a highly selective CSP for the separation of
only a single racemic product such as a drug. Since single enantiomers of that com-
pound must be prepared for the testing, the preparation of a “reciprocal” packing
with a single enantiomer attached to the support does not present a serious problem.
Once this CSP is available, a broad array of libraries of potential racemic selectors
can easily be screened. Although we selected a simple Biginelli three-component
condensation reaction to prepare the library of selectors based on “non-natural”
compounds, many other libraries of chiral organic compounds could also be
screened as potential selectors for the chiral recognition of specific targets. This
approach provides one more benefit: when such an extensive study is carried out
with structurally related families of compounds typical of chemical libraries, a bet-
ter understanding of chiral recognition may quickly be generated and used for design
of even more successful selectors.
3.7.3 Reciprocal Screening of Mixed Libraries
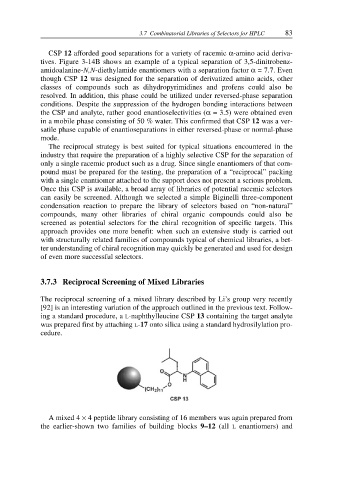
The reciprocal screening of a mixed library described by Li’s group very recently
[92] is an interesting variation of the approach outlined in the previous text. Follow-
ing a standard procedure, a L-naphthylleucine CSP 13 containing the target analyte
was prepared first by attaching L-17 onto silica using a standard hydrosilylation pro-
cedure.
A mixed 4 × 4 peptide library consisting of 16 members was again prepared from
the earlier-shown two families of building blocks 9–12 (all L enantiomers) and