Page 104 - Chiral Separation Techniques
P. 104
80 3 Combinatorial Approaches to Recognition of Chirality: Preparation …
6
5
4
Separation factor, α 3 2
1
0 R2
phenyl
4-isopropylphenyl 2,4-dimethylphenyl 3-methoxyphenyl 4-methoxyphenyl 2,3-dimethoxyphenyl 2,4-dimethoxyphenyl 3,4-methylenedioxyphenyl 3-hydroxyphenyl 2,4-dichlorophenyl 3-nitrophenyl 3,5-dinitrophenyl 2,4-dinitrophenyl 4-cyanophenyl 4-trifluoromethyl 2-thiophene 2-naphthyl 1-naphthyl 4-methoxy-1-naphthyl 1-pyrenyl 5-nitro-1-naphthyl 2-methoxy-
R1 Me
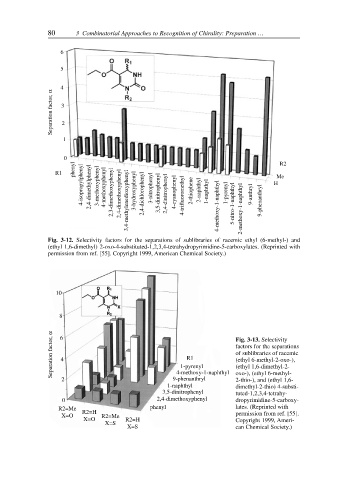
Fig. 3-12. Selectivity factors for the separations of sublibraries of racemic ethyl (6-methyl-) and
(ethyl 1,6-dimethyl) 2-oxo-4-substituted-1,2,3,4-tetrahydropyrimidine-5-carboxylates. (Reprinted with
permission from ref. [55]. Copyright 1999, American Chemical Society.)
10
8
Separation factor, α 6 4 1-pyrenyl Fig. 3-13. Selectivity
factors for the separations
of sublibraries of racemic
R1
(ethyl 6-methyl-2-oxo-),
(ethyl 1,6-dimethyl-2-
4-methoxy-1-naphthyl
2 9-phenanthryl oxo-), (ethyl 6-methyl-
2-thio-), and (ethyl 1,6-
1-naphthyl dimethyl-2-thio) 4-substi-
3,5-dinitrophenyl tuted-1,2,3,4-tetrahy-
0 2,4-dimethoxyphenyl dropyrimidine-5-carboxy-
R2=Me R2=H phenyl lates. (Reprinted with
X=O R2=Me permission from ref. [55].
X=O R2=H Copyright 1999, Ameri-
X=S
X=S can Chemical Society.)