Page 100 - Chiral Separation Techniques
P. 100
76 3 Combinatorial Approaches to Recognition of Chirality: Preparation …
The use of silica beads as a support for the preparation of peptide libraries appears
to be somewhat problematical. While Welch could demonstrate the very satisfactory
results described above [84–86], Li was not able to achieve complete coupling of the
first amino acid to all of the surface functional groups of modified silica, thus leav-
ing behind a number of functionalities that might be detrimental for the desired chi-
ral separations [87]. Therefore, Li’s group used the organic polymer supports that are
most common in the field of solid-phase synthesis: aminomethylated Merrifield
resin (2 % crosslinked polystyrene beads) and amine-terminated polyethylene glycol
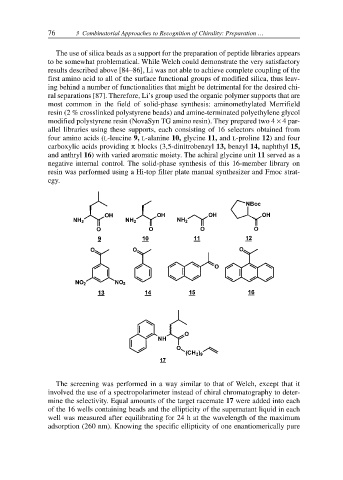
modified polystyrene resin (NovaSyn TG amino resin). They prepared two 4 × 4 par-
allel libraries using these supports, each consisting of 16 selectors obtained from
four amino acids (L-leucine 9, L-alanine 10, glycine 11, and L-proline 12) and four
carboxylic acids providing π blocks (3,5-dinitrobenzyl 13, benzyl 14, naphthyl 15,
and anthryl 16) with varied aromatic moiety. The achiral glycine unit 11 served as a
negative internal control. The solid-phase synthesis of this 16-member library on
resin was performed using a Hi-top filter plate manual synthesizer and Fmoc strat-
egy.
The screening was performed in a way similar to that of Welch, except that it
involved the use of a spectropolarimeter instead of chiral chromatography to deter-
mine the selectivity. Equal amounts of the target racemate 17 were added into each
of the 16 wells containing beads and the ellipticity of the supernatant liquid in each
well was measured after equilibrating for 24 h at the wavelength of the maximum
adsorption (260 nm). Knowing the specific ellipticity of one enantiomerically pure