Page 95 - Chiral Separation Techniques
P. 95
3.7 Combinatorial Libraries of Selectors for HPLC 71
3.7 Combinatorial Libraries of Selectors for HPLC
3.7.1 On-Bead Solid-Phase Synthesis of Chiral Dipeptides
Several attempts to prepare efficient chiral stationary phases using Merrifield’s
solid-phase peptide synthesis have been reported in the past. For example, in 1977
Gruska [82] prepared a tripeptide bound to a solid support using a sequence of pro-
tection – coupling – deprotection reactions. This approach appeared to suffer from
incomplete conversion in the coupling steps, and the stationary phase exhibited only
a modest selectivity. A similar stationary phase was later prepared by attaching the
pure tripeptide L-val-ala-pro, prepared in solution, to porous silica beads [83]. This
CSP exhibited higher selectivity than that of Gruska thus indicating the possible
detrimental effect of undesired or uncontrolled functionalities on the recognition
ability of the stationary phase. Recently, Welch analyzed these results and realized
that the primary reason for the relative failure of the early approaches to on-bead
solid-phase synthesis of oligopeptide selectors could be traced to the relatively low
reactivity of the functional silane reagent, 1-trimethoxysilyl-2-(4-chloromethyl-
phenyl)ethane, used for the preparation of the original chiral stationary phases [84].
–1
As a result of steric constrains, selector surface coverage of only 0.3 mmol g could
be achieved after activation using this bulky silane reagent. In contrast, Welch easily
obtained CSPs containing at least twice as much selector using aminopropyltri-
ethoxysilane activation. This group also optimized the reaction conditions to afford
silica beads with a high amine surface coverage and to realize their essentially quan-
titative functionalization in the subsequent reaction step. This more successful
approach enabled study of the effects of various variables on the separation proper-
ties of chiral stationary phases thus prepared.
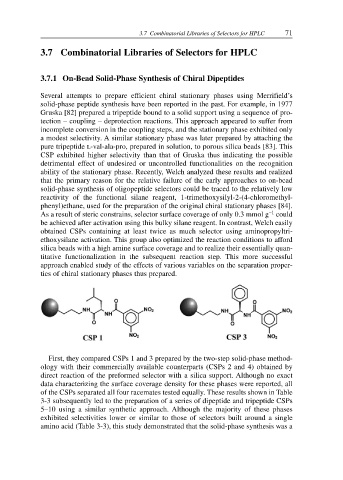
First, they compared CSPs 1 and 3 prepared by the two-step solid-phase method-
ology with their commercially available counterparts (CSPs 2 and 4) obtained by
direct reaction of the preformed selector with a silica support. Although no exact
data characterizing the surface coverage density for these phases were reported, all
of the CSPs separated all four racemates tested equally. These results shown in Table
3-3 subsequently led to the preparation of a series of dipeptide and tripeptide CSPs
5–10 using a similar synthetic approach. Although the majority of these phases
exhibited selectivities lower or similar to those of selectors built around a single
amino acid (Table 3-3), this study demonstrated that the solid-phase synthesis was a