Page 91 - Chiral Separation Techniques
P. 91
3.6 Library of Cyclic Oligopeptides as Additives to Background Electrolyte … 67
An example of the modular preparation of the cyclophane 3 from the substituted
bipyridine 2 and a general tripeptide 1 is shown in Scheme 3-3. The host molecule
3 contains a pre-organized binding pocket. The overall basicity of such molecules
also facilitates their intercalation within the lamellas of acidic zirconium phosphate,
thus making this chemistry well suited for the desired application.
While the bipyridinium part of the cycle is fixed, the peptidic module is amenable
to combinatorial variation. The ability of a library of cyclophanes 3 containing 20
dipeptides and one tripeptide to recognize chiral compounds was studied in deu-
1
terium oxide solutions using the facile H NMR titration technique that required
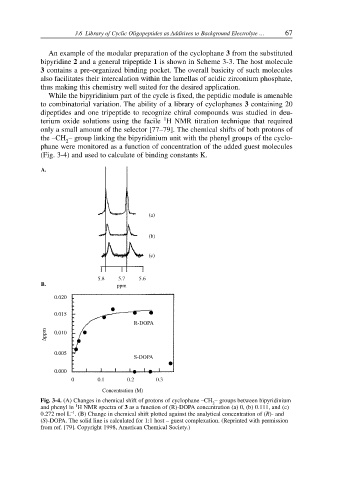
only a small amount of the selector [77–79]. The chemical shifts of both protons of
the –CH – group linking the bipyridinium unit with the phenyl groups of the cyclo-
2
phane were monitored as a function of concentration of the added guest molecules
(Fig. 3-4) and used to calculate of binding constants K.
A.
(a)
(b)
(c)
5.8 5.7 5.6
B. ppm
0.020
0.015
R-DOPA
∆ppm 0.010
0.005
S-DOPA
0.000
0 0.1 0.2 0.3
Concentration (M)
Fig. 3-4. (A) Changes in chemical shift of protons of cyclophane –CH – groups between bipyridinium
2
1
and phenyl in H NMR spectra of 3 as a function of (R)-DOPA concentration (a) 0, (b) 0.111, and (c)
–1
0.272 mol L . (B) Change in chemical shift plotted against the analytical concentration of (R)- and
(S)-DOPA. The solid line is calculated for 1:1 host – guest complexation. (Reprinted with permission
from ref. [79]. Copyright 1998, American Chemical Society.)