Page 90 - Chiral Separation Techniques
P. 90
66 3 Combinatorial Approaches to Recognition of Chirality: Preparation …
The improvements in resolution achieved in each deconvolution step are shown in
Figure 3-3. While the initial library could only afford a modest separation of DNB-
glutamic acid, the library with proline in position 4 also separated DNP derivatives
of alanine and aspartic acid, and further improvement in both resolution and the
number of separable racemates was observed for peptides with hydrophobic amino
acid residues in position 3. However, the most dramatic improvement and best selec-
tivity were found for c(Arg-Lys-Tyr-Pro-Tyr-β-Ala) (Scheme 3-2a) with the tyrosine
residue at position 5 with a resolution factor as high as 28 observed for the separa-
tion of DNP-glutamic acid enantiomers.
In addition to the development of the powerful chiral additive, this study also
demonstrated that the often tedious deconvolution process can be accelerated using
HPLC separation. As a result, only 15 libraries had to be synthesized instead of 64
libraries that would be required for the full-scale deconvolution. A somewhat simi-
lar approach also involving HPLC fractionations has recently been demonstrated by
Griffey for the deconvolution of libraries screened for biological activity [76].
Although demonstrated only for CE, the cyclic hexapeptides might also be useful
selectors for the preparation of chiral stationary phases for HPLC. However, this
would require the development of non-trivial additional chemistry to appropriately
link the peptide to a porous solid support.
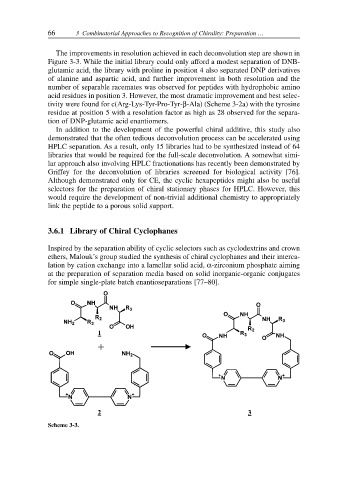
3.6.1 Library of Chiral Cyclophanes
Inspired by the separation ability of cyclic selectors such as cyclodextrins and crown
ethers, Malouk’s group studied the synthesis of chiral cyclophanes and their interca-
lation by cation exchange into a lamellar solid acid, α-zirconium phosphate aiming
at the preparation of separation media based on solid inorganic-organic conjugates
for simple single-plate batch enantioseparations [77–80].
Scheme 3-3.