Page 92 - Chiral Separation Techniques
P. 92
68 3 Combinatorial Approaches to Recognition of Chirality: Preparation …
For example, cyclophane 3 containing (L)-val-leu-ala tripeptide showed signifi-
cant association with (D)-3,4-dihydroxyphenylalanine 4 (DOPA) and the drug
–1
nadolol 5 with K values of 39 ± 6 and 23 ± 3 mol , respectively, demonstrating
rather high selectivity of this cyclic selector. In contrast, only very low binding con-
–1
stants were observed for (L)-DOPA (K = 3 mol ), D and L tryphtophan (K = 5 and
–1
–1
6 mol ), and N-2-naphthylalanine (K = 10 mol ) [79]. A further increase in the
hydrophobicity and, perhaps, enhanced π-acceptor ability of the lateral functionali-
ties of the oligopeptide moiety may help substantially to improve the selectivity that
would be required for their successful application in intercalated solids. It should be
noted that this category of selectors is exceptional since, in contrast to the vast
majority of typical selectors, it operates in environment friendly aqueous media.
Their solubility in water results from the cationic nature of the cyclophane.
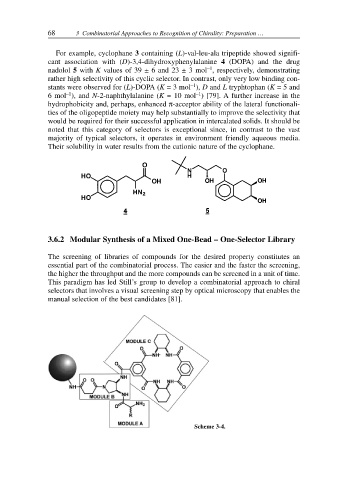
3.6.2 Modular Synthesis of a Mixed One-Bead – One-Selector Library
The screening of libraries of compounds for the desired property constitutes an
essential part of the combinatorial process. The easier and the faster the screening,
the higher the throughput and the more compounds can be screened in a unit of time.
This paradigm has led Still’s group to develop a combinatorial approach to chiral
selectors that involves a visual screening step by optical microscopy that enables the
manual selection of the best candidates [81].
Scheme 3-4.