Page 94 - Chiral Separation Techniques
P. 94
70 3 Combinatorial Approaches to Recognition of Chirality: Preparation …
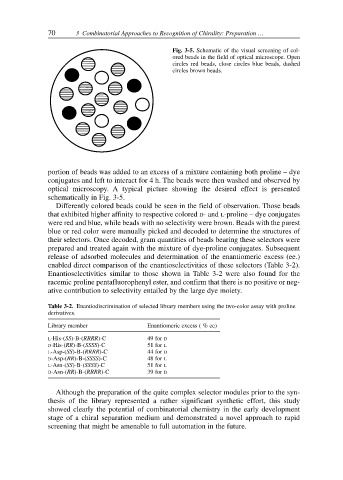
Fig. 3-5. Schematic of the visual screening of col-
ored beads in the field of optical microscope. Open
circles red beads, close circles blue beads, dashed
circles brown beads.
portion of beads was added to an excess of a mixture containing both proline – dye
conjugates and left to interact for 4 h. The beads were then washed and observed by
optical microscopy. A typical picture showing the desired effect is presented
schematically in Fig. 3-5.
Differently colored beads could be seen in the field of observation. Those beads
that exhibited higher affinity to respective colored D- and L-proline – dye conjugates
were red and blue, while beads with no selectivity were brown. Beads with the purest
blue or red color were manually picked and decoded to determine the structures of
their selectors. Once decoded, gram quantities of beads bearing these selectors were
prepared and treated again with the mixture of dye-proline conjugates. Subsequent
release of adsorbed molecules and determination of the enantiomeric excess (ee.)
enabled direct comparison of the enantioselectivities of these selectors (Table 3-2).
Enantioselectivities similar to those shown in Table 3-2 were also found for the
racemic proline pentafluorophenyl ester, and confirm that there is no positive or neg-
ative contribution to selectivity entailed by the large dye moiety.
Table 3-2. Enantiodiscrimination of selected library members using the two-color assay with proline
derivatives.
Library member Enantiomeric excess ( % ee)
L-His-(SS)-B-(RRRR)-C 49 for D
D-His-(RR)-B-(SSSS)-C 51 for L
L-Asp-(SS)-B-(RRRR)-C 44 for D
D-Asp-(RR)-B-(SSSS)-C 48 for L
L-Asn-(SS)-B-(SSSS)-C 51 for L
D-Asn-(RR)-B-(RRRR)-C 39 for D
Although the preparation of the quite complex selector modules prior to the syn-
thesis of the library represented a rather significant synthetic effort, this study
showed clearly the potential of combinatorial chemistry in the early development
stage of a chiral separation medium and demonstrated a novel approach to rapid
screening that might be amenable to full automation in the future.