Page 93 - Chiral Separation Techniques
P. 93
3.6 Library of Cyclic Oligopeptides as Additives to Background Electrolyte … 69
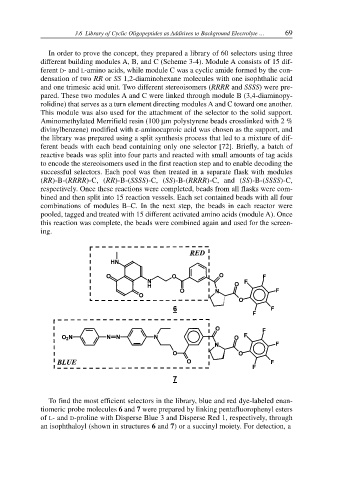
In order to prove the concept, they prepared a library of 60 selectors using three
different building modules A, B, and C (Scheme 3-4). Module A consists of 15 dif-
ferent D- and L-amino acids, while module C was a cyclic amide formed by the con-
densation of two RR or SS 1,2-diaminohexane molecules with one isophthalic acid
and one trimesic acid unit. Two different stereoisomers (RRRR and SSSS) were pre-
pared. These two modules A and C were linked through module B (3,4-diaminopy-
rolidine) that serves as a turn element directing modules A and C toward one another.
This module was also used for the attachment of the selector to the solid support.
Aminomethylated Merrifield resin (100 µm polystyrene beads crosslinked with 2 %
divinylbenzene) modified with ε-aminocaproic acid was chosen as the support, and
the library was prepared using a split synthesis process that led to a mixture of dif-
ferent beads with each bead containing only one selector [72]. Briefly, a batch of
reactive beads was split into four parts and reacted with small amounts of tag acids
to encode the stereoisomers used in the first reaction step and to enable decoding the
successful selectors. Each pool was then treated in a separate flask with modules
(RR)-B-(RRRR)-C, (RR)-B-(SSSS)-C, (SS)-B-(RRRR)-C, and (SS)-B-(SSSS)-C,
respectively. Once these reactions were completed, beads from all flasks were com-
bined and then split into 15 reaction vessels. Each set contained beads with all four
combinations of modules B–C. In the next step, the beads in each reactor were
pooled, tagged and treated with 15 different activated amino acids (module A). Once
this reaction was complete, the beads were combined again and used for the screen-
ing.
To find the most efficient selectors in the library, blue and red dye-labeled enan-
tiomeric probe molecules 6 and 7 were prepared by linking pentafluorophenyl esters
of L- and D-proline with Disperse Blue 3 and Disperse Red 1, respectively, through
an isophthaloyl (shown in structures 6 and 7) or a succinyl moiety. For detection, a