Page 105 - Chiral Separation Techniques
P. 105
3.7 Combinatorial Libraries of Selectors for HPLC 81
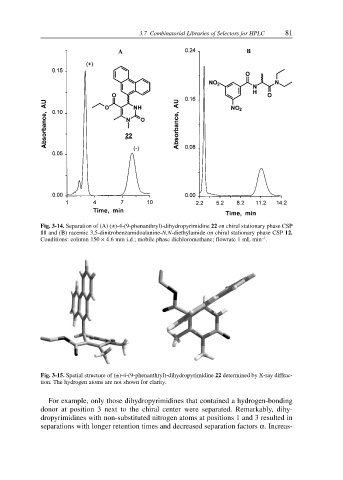
Fig. 3-14. Separation of (A) (±)-4-(9-phenanthryl)-dihydropyrimidine 22 on chiral stationary phase CSP
11 and (B) racemic 3,5-dinitrobenzamidoalanine-N,N-diethylamide on chiral stationary phase CSP 12.
–1
Conditions: column 150 × 4.6 mm i.d.; mobile phase dichloromethane; flowrate 1 mL min .
Fig. 3-15. Spatial structure of (±)-4-(9-phenanthryl)-dihydropyrimidine 22 determined by X-ray diffrac-
tion. The hydrogen atoms are not shown for clarity.
For example, only those dihydropyrimidines that contained a hydrogen-bonding
donor at position 3 next to the chiral center were separated. Remarkably, dihy-
dropyrimidines with non-substituted nitrogen atoms at positions 1 and 3 resulted in
separations with longer retention times and decreased separation factors α. Increas-