Page 108 - Chiral Separation Techniques
P. 108
84 3 Combinatorial Approaches to Recognition of Chirality: Preparation …
13–16 shown earlier, each terminated with a glycine unit, using a solid-phase syn-
thesis followed by cleavage. This complete library was injected on a short column
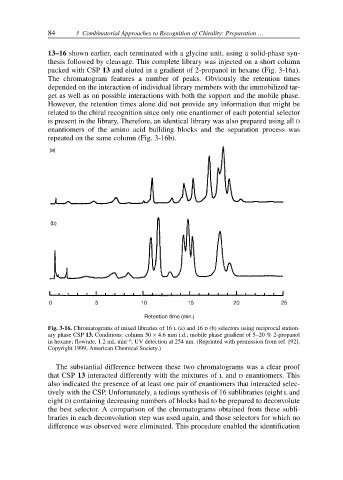
packed with CSP 13 and eluted in a gradient of 2-propanol in hexane (Fig. 3-16a).
The chromatogram features a number of peaks. Obviously the retention times
depended on the interaction of individual library members with the immobilized tar-
get as well as on possible interactions with both the support and the mobile phase.
However, the retention times alone did not provide any information that might be
related to the chiral recognition since only one enantiomer of each potential selector
is present in the library. Therefore, an identical library was also prepared using all D
enantiomers of the amino acid building blocks and the separation process was
repeated on the same column (Fig. 3-16b).
Fig. 3-16. Chromatograms of mixed libraries of 16 L (a) and 16 D (b) selectors using reciprocal station-
ary phase CSP 13. Conditions: column 50 × 4.6 mm i.d.; mobile phase gradient of 5–20 % 2-propanol
–1
in hexane; flowrate, 1.2 mL min ; UV detection at 254 nm. (Reprinted with permission from ref. [92].
Copyright 1999, American Chemical Society.)
The substantial difference between these two chromatograms was a clear proof
that CSP 13 interacted differently with the mixtures of L and D enantiomers. This
also indicated the presence of at least one pair of enantiomers that interacted selec-
tively with the CSP. Unfortunately, a tedious synthesis of 16 sublibraries (eight L and
eight D) containing decreasing numbers of blocks had to be prepared to deconvolute
the best selector. A comparison of the chromatograms obtained from these subli-
braries in each deconvolution step was used again, and those selectors for which no
difference was observed were eliminated. This procedure enabled the identification