Page 110 - Chiral Separation Techniques
P. 110
86 3 Combinatorial Approaches to Recognition of Chirality: Preparation …
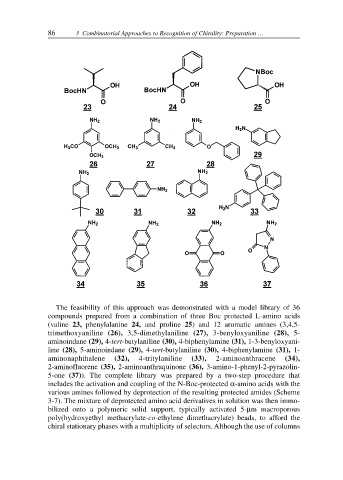
The feasibility of this approach was demonstrated with a model library of 36
compounds prepared from a combination of three Boc protected L-amino acids
(valine 23, phenylalanine 24, and proline 25) and 12 aromatic amines (3,4,5-
trimethoxyaniline (26), 3,5-dimethylaniline (27), 3-benyloxyaniline (28), 5-
aminoindane (29), 4-tert-butylaniline (30), 4-biphenylamine (31), 1-3-benyloxyani-
line (28), 5-aminoindane (29), 4-tert-butylaniline (30), 4-biphenylamine (31), 1-
aminonaphthalene (32), 4-tritylaniline (33), 2-aminoanthracene (34),
2-aminofluorene (35), 2-aminoanthraquinone (36), 3-amino-1-phenyl-2-pyrazolin-
5-one (37)). The complete library was prepared by a two-step procedure that
includes the activation and coupling of the N-Boc-protected α-amino acids with the
various amines followed by deprotection of the resulting protected amides (Scheme
3-7). The mixture of deprotected amino acid derivatives in solution was then immo-
bilized onto a polymeric solid support, typically activated 5-µm macroporous
poly(hydroxyethyl methacrylate-co-ethylene dimethacrylate) beads, to afford the
chiral stationary phases with a multiplicity of selectors. Although the use of columns