Page 106 - Chiral Separation Techniques
P. 106
82 3 Combinatorial Approaches to Recognition of Chirality: Preparation …
ing the π-basicity of the aromatic group at C4 resulted in higher separation factors
due to a stronger interaction with the π-acidic 3,5-dinitrobenzoyl group of CSP 11
used for the screening. Obviously, the substitution pattern as well as the π-basicity
of the aromatic group at the C4 atom play essential roles. Dihydropyrimidines with
ortho-substituted aromatic groups show much higher enantioselectivities compared
to meta- and para-substituted groups. For example, the observed enantioselectivity
for the 1-naphthyl-dihydropyrimidines was almost twice that of the corresponding 2-
naphthyl derivative (see Fig. 3-12). Addition of a second ortho substituent in the aro-
matic ring of the dihydropyrimidines led to a dramatic deterioration of enantiosepa-
ration as observed with the 9-anthryl or 2-methoxy-1-naphthyl substituted com-
pounds. Finally, another important effect on enantioselectivity was observed when a
thiourea was used instead of urea for the preparation of 21. In general, the selectiv-
ity factor for the 2-thio analogs increased two-fold compared to the corresponding
2-oxo-DHPMs (see Fig. 3-13). For example, 4-(9-phenanthryl)-2-thiodihydropyri-
midine exhibited a selectivity of α = 11.7 instead of 5.2 for the best oxygenated ana-
logue. However, the lability of the thio-containing compounds compared to the oxo-
analogue led us to select the latter for the preparation of more rugged CSPs.
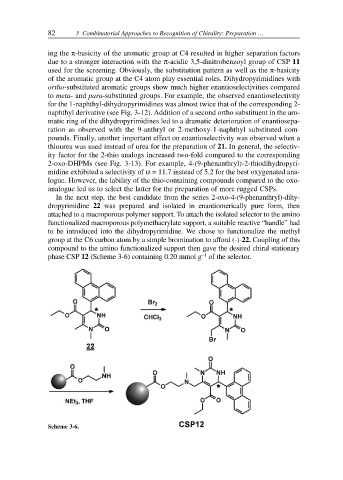
In the next step, the best candidate from the series 2-oxo-4-(9-phenanthryl)-dihy-
dropyrimidine 22 was prepared and isolated in enantiomerically pure form, then
attached to a macroporous polymer support. To attach the isolated selector to the amino
functionalized macroporous polymethacrylate support, a suitable reactive “handle” had
to be introduced into the dihydropyrimidine. We chose to functionalize the methyl
group at the C6 carbon atom by a simple bromination to afford (-)-22. Coupling of this
compound to the amino functionalized support then gave the desired chiral stationary
–1
phase CSP 12 (Scheme 3-6) containing 0.20 mmol g of the selector.
Scheme 3-6.