Page 103 - Chiral Separation Techniques
P. 103
3.7 Combinatorial Libraries of Selectors for HPLC 79
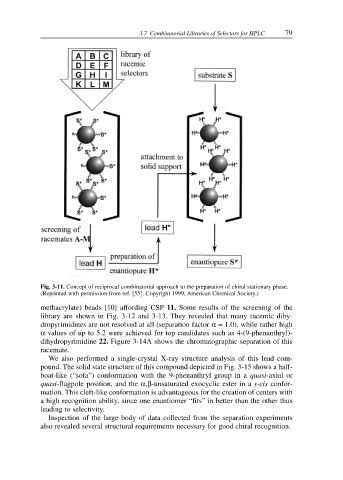
Fig. 3-11. Concept of reciprocal combinatorial approach to the preparation of chiral stationary phase.
(Reprinted with permission from ref. [55]. Copyright 1999, American Chemical Society.)
methacrylate) beads [10] affording CSP 11. Some results of the screening of the
library are shown in Fig. 3-12 and 3-13. They revealed that many racemic dihy-
dropyrimidines are not resolved at all (separation factor α = 1.0), while rather high
α values of up to 5.2 were achieved for top candidates such as 4-(9-phenanthryl)-
dihydropyrimidine 22. Figure 3-14A shows the chromatographic separation of this
racemate.
We also performed a single-crystal X-ray structure analysis of this lead com-
pound. The solid state structure of this compound depicted in Fig. 3-15 shows a half-
boat-like (“sofa”) conformation with the 9-phenanthryl group in a quasi-axial or
quasi-flagpole position, and the α,β-unsaturated exocyclic ester in a s-cis confor-
mation. This cleft-like conformation is advantageous for the creation of centers with
a high recognition ability, since one enantiomer “fits” in better than the other thus
leading to selectivity.
Inspection of the large body of data collected from the separation experiments
also revealed several structural requirements necessary for good chiral recognition.