Page 102 - Chiral Separation Techniques
P. 102
78 3 Combinatorial Approaches to Recognition of Chirality: Preparation …
3.7.2 Reciprocal Screening of Parallel Library
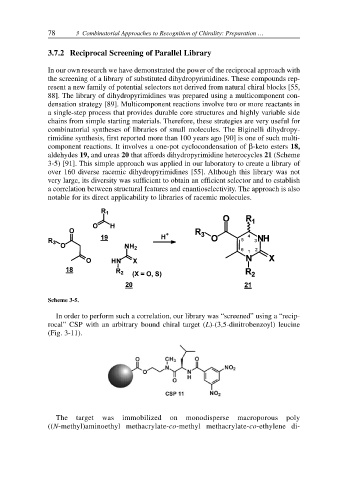
In our own research we have demonstrated the power of the reciprocal approach with
the screening of a library of substituted dihydropyrimidines. These compounds rep-
resent a new family of potential selectors not derived from natural chiral blocks [55,
88]. The library of dihydropyrimidines was prepared using a multicomponent con-
densation strategy [89]. Multicomponent reactions involve two or more reactants in
a single-step process that provides durable core structures and highly variable side
chains from simple starting materials. Therefore, these strategies are very useful for
combinatorial syntheses of libraries of small molecules. The Biginelli dihydropy-
rimidine synthesis, first reported more than 100 years ago [90] is one of such multi-
component reactions. It involves a one-pot cyclocondensation of β-keto esters 18,
aldehydes 19, and ureas 20 that affords dihydropyrimidine heterocycles 21 (Scheme
3-5) [91]. This simple approach was applied in our laboratory to create a library of
over 160 diverse racemic dihydropyrimidines [55]. Although this library was not
very large, its diversity was sufficient to obtain an efficient selector and to establish
a correlation between structural features and enantioselectivity. The approach is also
notable for its direct applicability to libraries of racemic molecules.
Scheme 3-5.
In order to perform such a correlation, our library was “screened” using a “recip-
rocal” CSP with an arbitrary bound chiral target (L)-(3,5-dinitrobenzoyl) leucine
(Fig. 3-11).
The target was immobilized on monodisperse macroporous poly
((N-methyl)aminoethyl methacrylate-co-methyl methacrylate-co-ethylene di-