Page 132 - Chiral Separation Techniques
P. 132
4.5 3D Structure Database Searches 109
One may be surprised by the simplicity of those enantiophore queries. Neverthe-
less, in respect of the stereochemical constraints, such combinations of interactions
involving hydrogen-bond donor or acceptor groups and π-stacking are sufficiently
specific and directional to provide adequate chiral recognition models. A certain tol-
erance has been attached to the distances (± 0.5Å) or angles because a precise coin-
cidence of atoms is not necessary as specific interactions may take place over a range
of angles and distances.
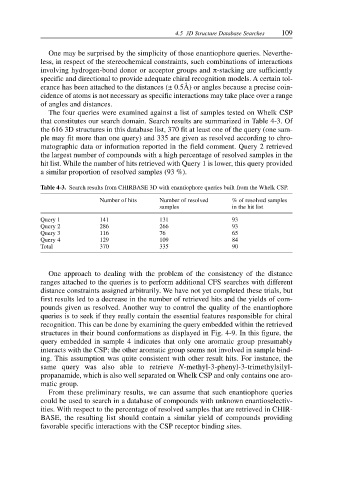
The four queries were examined against a list of samples tested on Whelk CSP
that constitutes our search domain. Search results are summarized in Table 4-3. Of
the 616 3D structures in this database list, 370 fit at least one of the query (one sam-
ple may fit more than one query) and 335 are given as resolved according to chro-
matographic data or information reported in the field comment. Query 2 retrieved
the largest number of compounds with a high percentage of resolved samples in the
hit list. While the number of hits retrieved with Query 1 is lower, this query provided
a similar proportion of resolved samples (93 %).
Table 4-3. Search results from CHIRBASE 3D with enantiophore queries built from the Whelk CSP.
Number of hits Number of resolved % of resolved samples
samples in the hit list
Query 1 141 131 93
Query 2 286 266 93
Query 3 116 76 65
Query 4 129 109 84
Total 370 335 90
One approach to dealing with the problem of the consistency of the distance
ranges attached to the queries is to perform additional CFS searches with different
distance constraints assigned arbitrarily. We have not yet completed these trials, but
first results led to a decrease in the number of retrieved hits and the yields of com-
pounds given as resolved. Another way to control the quality of the enantiophore
queries is to seek if they really contain the essential features responsible for chiral
recognition. This can be done by examining the query embedded within the retrieved
structures in their bound conformations as displayed in Fig. 4-9. In this figure, the
query embedded in sample 4 indicates that only one aromatic group presumably
interacts with the CSP; the other aromatic group seems not involved in sample bind-
ing. This assumption was quite consistent with other result hits. For instance, the
same query was also able to retrieve N-methyl-3-phenyl-3-trimethylsilyl-
propanamide, which is also well separated on Whelk CSP and only contains one aro-
matic group.
From these preliminary results, we can assume that such enantiophore queries
could be used to search in a database of compounds with unknown enantioselectiv-
ities. With respect to the percentage of resolved samples that are retrieved in CHIR-
BASE, the resulting list should contain a similar yield of compounds providing
favorable specific interactions with the CSP receptor binding sites.