Page 134 - Chiral Separation Techniques
P. 134
4.5 3D Structure Database Searches 111
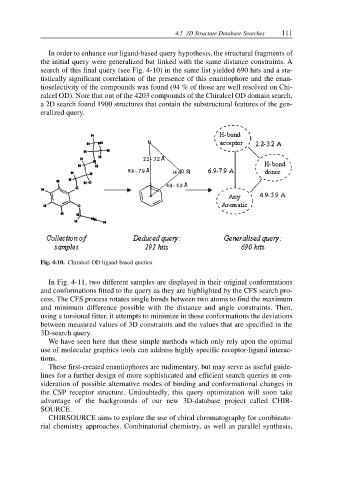
In order to enhance our ligand-based query hypothesis, the structural fragments of
the initial query were generalized but linked with the same distance constraints. A
search of this final query (see Fig. 4-10) in the same list yielded 690 hits and a sta-
tistically significant correlation of the presence of this enantiophore and the enan-
tioselectivity of the compounds was found (94 % of those are well resolved on Chi-
ralcel OD). Note that out of the 4203 compounds of the Chiralcel OD domain search,
a 2D search found 1900 structures that contain the substructural features of the gen-
eralized query.
Fig. 4-10. Chiralcel OD ligand-based queries.
In Fig. 4-11, two different samples are displayed in their original conformations
and conformations fitted to the query as they are highlighted by the CFS search pro-
cess. The CFS process rotates single bonds between two atoms to find the maximum
and minimum difference possible with the distance and angle constraints. Then,
using a torsional fitter, it attempts to minimize in those conformations the deviations
between measured values of 3D constraints and the values that are specified in the
3D-search query.
We have seen here that these simple methods which only rely upon the optimal
use of molecular graphics tools can address highly specific receptor-ligand interac-
tions.
These first-created enantiophores are rudimentary, but may serve as useful guide-
lines for a further design of more sophisticated and efficient search queries in con-
sideration of possible alternative modes of binding and conformational changes in
the CSP receptor structure. Undoubtedly, this query optimization will soon take
advantage of the backgrounds of our new 3D-database project called CHIR-
SOURCE.
CHIRSOURCE aims to explore the use of chiral chromatography for combinato-
rial chemistry approaches. Combinatorial chemistry, as well as parallel synthesis,