Page 135 - Chiral Separation Techniques
P. 135
112 4 CHIRBASE: Database Current Status and Derived Research Applications using …
requires the availability of both enantiomers to address the configurational diversity
issues. The availability of both enantiomers is not so common as far as the sources
come from the « chiral pool » or implies two separated asymmetric synthesis or res-
olution, which often require further enantiomeric purification. Preparative (or
semipreparative) chiral liquid chromatography is the method of choice for the avail-
ability of both enantiomers in high enantiomeric purity in a single shot. Simulated
moving bed technology is available for larger-scale separations.
Designed from CHIRBASE-3D, CHIRSOURCE provides 30 000 structures in
terms of configurational diversity, most of them easily available by semipreparative
scale on corporate installation or in dedicated companies with minor further opti-
mization.
We are today persuaded that CHIRSOURCE can help to reduce the costs and
means that are required to launch a new chiral drug to market. CHIRSOURCE will
include molecular attributes (dipole, lipophilicity, surface area and volume, HOMO-
LUMO, Verloop parameters, molar refractivity) and molecular indices (describing
connectivity, shape, topology and electrotopology, atom, ring and group counts).
Such 3D molecular descriptors are often used in cluster analysis to identify dissim-
ilar compounds for combinatorial chemistry and high-throughput screening applica-
tions.
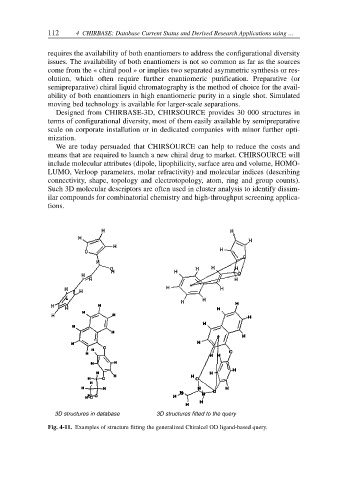
3D structures in database 3D structures fitted to the query
Fig. 4-11. Examples of structure fitting the generalized Chiralcel OD ligand-based query.