Page 153 - Chiral Separation Techniques
P. 153
5.2 Chiral Membranes 131
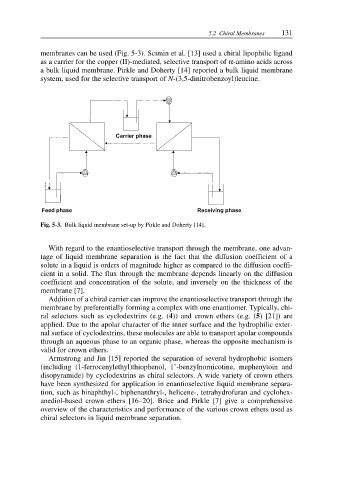
membranes can be used (Fig. 5-3). Scimin et al. [13] used a chiral lipophilic ligand
as a carrier for the copper (II)-mediated, selective transport of α-amino acids across
a bulk liquid membrane. Pirkle and Doherty [14] reported a bulk liquid membrane
system, used for the selective transport of N-(3,5-dinitrobenzoyl)leucine.
Fig. 5-3. Bulk liquid membrane set-up by Pirkle and Doherty [14].
With regard to the enantioselective transport through the membrane, one advan-
tage of liquid membrane separation is the fact that the diffusion coefficient of a
solute in a liquid is orders of magnitude higher as compared to the diffusion coeffi-
cient in a solid. The flux through the membrane depends linearly on the diffusion
coefficient and concentration of the solute, and inversely on the thickness of the
membrane [7].
Addition of a chiral carrier can improve the enantioselective transport through the
membrane by preferentially forming a complex with one enantiomer. Typically, chi-
ral selectors such as cyclodextrins (e.g. (4)) and crown ethers (e.g. (5) [21]) are
applied. Due to the apolar character of the inner surface and the hydrophilic exter-
nal surface of cyclodextrins, these molecules are able to transport apolar compounds
through an aqueous phase to an organic phase, whereas the opposite mechanism is
valid for crown ethers.
Armstrong and Jin [15] reported the separation of several hydrophobic isomers
(including (1-ferrocenylethyl)thiophenol, 1’-benzylnornicotine, mephenytoin and
disopyramide) by cyclodextrins as chiral selectors. A wide variety of crown ethers
have been synthesized for application in enantioselective liquid membrane separa-
tion, such as binaphthyl-, biphenanthryl-, helicene-, tetrahydrofuran and cyclohex-
anediol-based crown ethers [16–20]. Brice and Pirkle [7] give a comprehensive
overview of the characteristics and performance of the various crown ethers used as
chiral selectors in liquid membrane separation.