Page 155 - Chiral Separation Techniques
P. 155
5.2 Chiral Membranes 133
type of polymer membrane requires a high specific surface, low mass transfer resis-
tance, good mechanical strength and enantio recognition ability [23]. The separation
mechanism involves enantiospecific interactions (solution and diffusion) between
the isomers to be separated and the top layer polymer matrix. In case the required
optical purity cannot be obtained in one single step, a cascade of membrane units can
easily be applied to achieve the desired purity (see Section 5.2.4). Both the perme-
ability P (flux) and enantioselectivity (α) determine the performance of an enan-
tioselective membrane, for which α is defined as the ratio of the permeabilities of
the L- and D-enantiomers, respectively:
α= P L (1)
P
D
Enantiospecific polymers commonly used as stationary phases in chromatography
are potentially applicable for chiral membrane separation, e.g. polysaccharides,
acrylic polymers, poly(α-amino acids) and polyacetylene-derived polymers [24].
Additionally, chiral separations have been reported at high resolution and high rate
by a bovine serum albumin (BSA)-multilayer-adsorbed porous hollow-fiber mem-
brane as stationary phase [25]. In general, interest has been mainly focused on the
separation of racemic amino acids by enantioselective polymer membranes [26–35].
Although almost complete resolution can be obtained in a dialysis configuration, the
flux through this type of membranes is extremely low. Aoki and co-workers [36–41]
reported the preparation of membranes completely made of chiral polymer in order
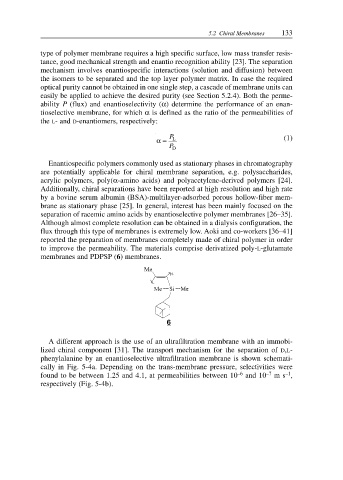
to improve the permeability. The materials comprise derivatized poly-L-glutamate
membranes and PDPSP (6) membranes.
A different approach is the use of an ultrafiltration membrane with an immobi-
lized chiral component [31]. The transport mechanism for the separation of D,L-
phenylalanine by an enantioselective ultrafiltration membrane is shown schemati-
cally in Fig. 5-4a. Depending on the trans-membrane pressure, selectivities were
–1
found to be between 1.25 and 4.1, at permeabilities between 10 –6 and 10 –7 m s ,
respectively (Fig. 5-4b).