Page 186 - Chiral Separation Techniques
P. 186
164 6 Enantiomer Separations using Designed Imprinted Chiral Phases
isotherms for L-PA were slightly better fitted to a Freundlich isotherm model, par-
ticularly at low temperatures. However, at concentrations higher than 17 µm (4 ×
–3
–1
10 g L ), the isotherm data of L-PA were equally well fitted to the Freundlich and
to the bi-Langmuir isotherm models, suggesting the existence of binding sites with
–1
higher binding energies (K > 50 000 M ). At 40 °C for L-PA, the binding constants
1
and site densities are respectively 84 M –1 and ca. 90 µmol g– for the low-affinity
–1
–1
sites, and 16 000 M and 1 µmol g for the high-affinity sites. For D-PA the respec-
–1
–1
–1
tive values are 48 M and 136 µmol g for the low-affinity sites, and 5520 M and
0.4 µmol g –1 for the high-affinity sites. These values agree well with those deter-
mined in previous studies [18]. In view of the small saturation capacities observed
for D-PA on these sites at the other temperatures studied (50, 60, 70 °C) or after ther-
mal annealing of the materials [24], the second site class appears to be specific for
L-PA.
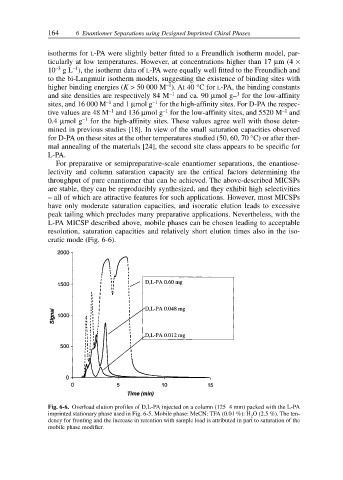
For preparative or semipreparative-scale enantiomer separations, the enantiose-
lectivity and column saturation capacity are the critical factors determining the
throughput of pure enantiomer that can be achieved. The above-described MICSPs
are stable, they can be reproducibly synthesized, and they exhibit high selectivities
– all of which are attractive features for such applications. However, most MICSPs
have only moderate saturation capacities, and isocratic elution leads to excessive
peak tailing which precludes many preparative applications. Nevertheless, with the
L-PA MICSP described above, mobile phases can be chosen leading to acceptable
resolution, saturation capacities and relatively short elution times also in the iso-
cratic mode (Fig. 6-6).
Fig. 6-6. Overload elution profiles of D,L-PA injected on a column (125 4 mm) packed with the L-PA
imprinted stationary phase used in Fig. 6-5. Mobile phase: MeCN: TFA (0.01 %): H O (2.5 %). The ten-
2
dency for fronting and the increase in retention with sample load is attributed in part to saturation of the
mobile phase modifier.