Page 190 - Chiral Separation Techniques
P. 190
168 6 Enantiomer Separations using Designed Imprinted Chiral Phases
ation strength for a given acid increases with the basicity of the base [57]. Thus, tem-
plates containing Brönsted-basic or hydrogen-bonding functional groups are poten-
tially suitable templates for the MAA/EDMA system [15]. Furthermore, more stable
cyclic hydrogen bonds can form with templates containing acid [27], amide[26] or
functionalized nitrogen heterocycles [39, 44]. The potential for a given monomer
template pair to produce templated sites can be predicted by measuring the stability
constants, e.g. by spectroscopic techniques, in a homogeneous solution mimicking
the monomer mixture prior to polymerization [15]. This can ultimately be used as a
preliminary screening procedure to search for suitable functional monomers. Thus,
estimated solution association constants can be correlated with the heterogeneous
binding constants determined for the polymer (Table 6-4). For the prepolymerization
complexes discussed thus far, the electrostatic interactions are sensitive to the pres-
ence of polar protic solvents. One exception is the complex formed between car-
boxylic acids and guanines or amidines [58, 59]. Here, cyclic hydrogen-bonded ion-
pairs are formed with stability constants that are order of magnitude higher than
those previously discussed (Table 6-4). This allows amidines such as pentamidine
(12) to be imprinted using iso-propanol–water as a porogenic solvent mixture,
resulting in polymers that bind pentamidine strongly in aqueous media [60, 90].
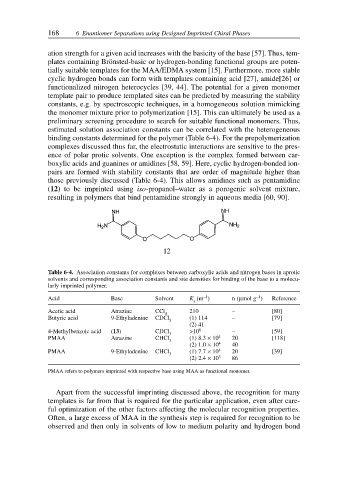
Table 6-4. Association constants for complexes between carboxylic acids and nitrogen bases in aprotic
solvents and corresponding association constants and site densities for binding of the base to a molecu-
larly imprinted polymer.
–1
–1
Acid Base Solvent K (m ) n (µmol g ) Reference
a
Acetic acid Atrazine CCl 210 – [80]
4
Butyric acid 9-Ethyladenine CDCl 3 (1) 114 – [79]
(2) 41
4-Methylbenzoic acid (13) CDCl 3 >10 6 – [59]
PMAA Atrazine CHCl (1) 8.3 × 10 4 20 [118]
3
(2) 1.0 × 10 4 40
PMAA 9-Ethyladenine CHCl (1) 7.7 × 10 4 20 [39]
3
(2) 2.4 × 10 3 86
PMAA refers to polymers imprinted with respective base using MAA as functional monomer.
Apart from the successful imprinting discussed above, the recognition for many
templates is far from that is required for the particular application, even after care-
ful optimization of the other factors affecting the molecular recognition properties.
Often, a large excess of MAA in the synthesis step is required for recognition to be
observed and then only in solvents of low to medium polarity and hydrogen bond