Page 191 - Chiral Separation Techniques
P. 191
6.5 Adsorption–Desorption Kinetics and Chromatographic Band Broadening 169
capacity [61]. In fact, in these cases the optimum rebinding solvent is often the sol-
vent used as porogen.[62] Thus, the polymer exhibits memory for the template as
well as the porogen. Moreover, the excess of functional monomer results in a por-
tion of the functional monomer not being associated with imprinted sites. These sites
interact nonselectively with solutes binding to carboxylic acids and limit the degree
of separation that can be achieved. Hence MAA is not a universal monomer. Instead,
for the recognition of any given target molecule access to functional monomers tar-
geted towards structural features, specific for particular compounds or classes of
compounds are required.
Based on the structural features of the templates that generate good sites, an inter-
esting possibility would be to incorporate these structures in new functional
monomers for the recognition of carboxylic acids. This concept is somewhat similar
to the reciprocity concept in the design of chiral stationary phases [63]. Thus, Wulff
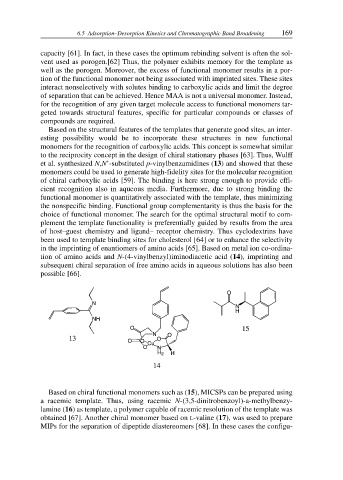
et al. synthesized N,N’-substituted p-vinylbenzamidines (13) and showed that these
monomers could be used to generate high-fidelity sites for the molecular recognition
of chiral carboxylic acids [59]. The binding is here strong enough to provide effi-
cient recognition also in aqueous media. Furthermore, due to strong binding the
functional monomer is quantitatively associated with the template, thus minimizing
the nonspecific binding. Functional group complementarity is thus the basis for the
choice of functional monomer. The search for the optimal structural motif to com-
plement the template functionality is preferentially guided by results from the area
of host–guest chemistry and ligand– receptor chemistry. Thus cyclodextrins have
been used to template binding sites for cholesterol [64] or to enhance the selectivity
in the imprinting of enantiomers of amino acids [65]. Based on metal ion co-ordina-
tion of amino acids and N-(4-vinylbenzyl)iminodiacetic acid (14), imprinting and
subsequent chiral separation of free amino acids in aqueous solutions has also been
possible [66].
Based on chiral functional monomers such as (15), MICSPs can be prepared using
a racemic template. Thus, using racemic N-(3,5-dinitrobenzoyl)-a-methylbenzy-
lamine (16) as template, a polymer capable of racemic resolution of the template was
obtained [67]. Another chiral monomer based on L-valine (17), was used to prepare
MIPs for the separation of dipeptide diastereomers [68]. In these cases the configu-