Page 195 - Chiral Separation Techniques
P. 195
6.5 Adsorption–Desorption Kinetics and Chromatographic Band Broadening 173
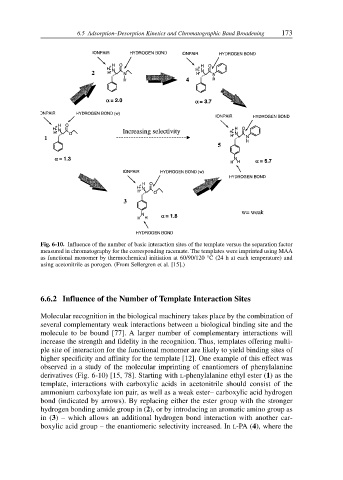
Fig. 6-10. Influence of the number of basic interaction sites of the template versus the separation factor
measured in chromatography for the corresponding racemate. The templates were imprinted using MAA
as functional monomer by thermochemical initiation at 60/90/120 °C (24 h at each temperature) and
using acetonitrile as porogen. (From Sellergren et al. [15].)
6.6.2 Influence of the Number of Template Interaction Sites
Molecular recognition in the biological machinery takes place by the combination of
several complementary weak interactions between a biological binding site and the
molecule to be bound [77]. A larger number of complementary interactions will
increase the strength and fidelity in the recognition. Thus, templates offering multi-
ple site of interaction for the functional monomer are likely to yield binding sites of
higher specificity and affinity for the template [12]. One example of this effect was
observed in a study of the molecular imprinting of enantiomers of phenylalanine
derivatives (Fig. 6-10) [15, 78]. Starting with L-phenylalanine ethyl ester (1) as the
template, interactions with carboxylic acids in acetonitrile should consist of the
ammonium carboxylate ion pair, as well as a weak ester– carboxylic acid hydrogen
bond (indicated by arrows). By replacing either the ester group with the stronger
hydrogen bonding amide group in (2), or by introducing an aromatic amino group as
in (3) – which allows an additional hydrogen bond interaction with another car-
boxylic acid group – the enantiomeric selectivity increased. In L-PA (4), where the