Page 194 - Chiral Separation Techniques
P. 194
172 6 Enantiomer Separations using Designed Imprinted Chiral Phases
order as those obtained using MAA for basic templates [73, 74]. These polymers are,
however, susceptible to oxidative degradation and require special handling.
In the imprinting of carboxylic acids and amides, high selectivities are also seen
using acrylamide (AAM) as functional monomer [28]. Furthermore, combinations of
two or more functional monomers, giving terpolymers or higher polymers, have in a
number of cases resulted in better recognition ability than the recognition observed
from the corresponding co-polymers [67, 73-75]. These systems are particularly
complex when the monomers constitute a donor–acceptor pair, since
monomer–monomer association will compete strongly with template–monomer
association if neither of the monomers has a particular preference for the template.
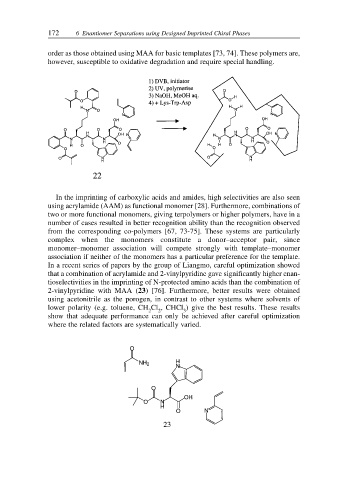
In a recent series of papers by the group of Liangmo, careful optimization showed
that a combination of acrylamide and 2-vinylpyridine gave significantly higher enan-
tioselectivities in the imprinting of N-protected amino acids than the combination of
2-vinylpyridine with MAA (23) [76]. Furthermore, better results were obtained
using acetonitrile as the porogen, in contrast to other systems where solvents of
lower polarity (e.g. toluene, CH Cl , CHCl ) give the best results. These results
2 2 3
show that adequate performance can only be achieved after careful optimization
where the related factors are systematically varied.