Page 182 - Chiral Separation Techniques
P. 182
160 6 Enantiomer Separations using Designed Imprinted Chiral Phases
smaller substituents, the recognition is not purely size exclusion but instead must be
driven by shape complementarity between the site and the substrate, or conforma-
1
tional differences between the derivatives. It was concluded on the basis of H-NMR
nuclear Overhauser enhancement experiments and molecular mechanics calculations
that L-PA and the N-methylanilide exhibit large conformational differences. Thus,
the torsional angles between the anilide ring plane and the amide plane, as well as in
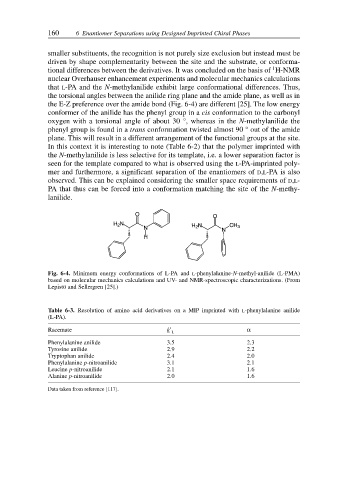
the E-Z preference over the amide bond (Fig. 6-4) are different [25]. The low energy
conformer of the anilide has the phenyl group in a cis conformation to the carbonyl
oxygen with a torsional angle of about 30 °, whereas in the N-methylanilide the
phenyl group is found in a trans conformation twisted almost 90 ° out of the amide
plane. This will result in a different arrangement of the functional groups at the site.
In this context it is interesting to note (Table 6-2) that the polymer imprinted with
the N-methylanilide is less selective for its template, i.e. a lower separation factor is
seen for the template compared to what is observed using the L-PA-imprinted poly-
mer and furthermore, a significant separation of the enantiomers of D,L-PA is also
observed. This can be explained considering the smaller space requirements of D,L-
PA that thus can be forced into a conformation matching the site of the N-methy-
lanilide.
Fig. 6-4. Minimum energy conformations of L-PA and L-phenylalanine-N-methyl-anilide (L-PMA)
based on molecular mechanics calculations and UV- and NMR-spectroscopic characterizations. (From
Lepistö and Sellergren [25].)
Table 6-3. Resolution of amino acid derivatives on a MIP imprinted with L-phenylalanine anilide
(L-PA).
Racemate k’ L α
Phenylalanine anilide 3.5 2.3
Tyrosine anilide 2.9 2.2
Tryptophan anilide 2.4 2.0
Phenylalanine p-nitroanilide 3.1 2.1
Leucine p-nitroanilide 2.1 1.6
Alanine p-nitroanilide 2.0 1.6
Data taken from reference [117].