Page 178 - Chiral Separation Techniques
P. 178
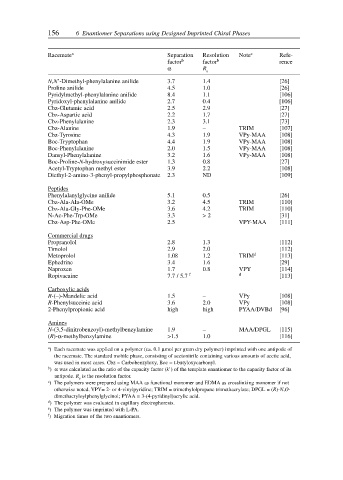
156 6 Enantiomer Separations using Designed Imprinted Chiral Phases
Racemate a Separation Resolution Note c Refe-
factor b factor b rence
α R s
N,N’-Dimethyl-phenylalanine anilide 3.7 1.4 [26]
Proline anilide 4.5 1.0 [26]
Pyridylmethyl-phenylalanine anilide 8.4 1.1 [106]
Pyridoxyl-phenylalanine anilide 2.7 0.4 [106]
Cbz-Glutamic acid 2.5 2.9 [27]
Cbz-Aspartic acid 2.2 1.7 [27]
Cbz-Phenylalanine 2.3 3.1 [73]
Cbz-Alanine 1.9 – TRIM [107]
Cbz-Tyrosine 4.3 1.9 VPy-MAA [108]
Boc-Tryptophan 4.4 1.9 VPy-MAA [108]
Boc-Phenylalanine 2.0 1.5 VPy-MAA [108]
Dansyl-Phenylalanine 3.2 1.6 VPy-MAA [108]
Boc-Proline-N-hydroxysuccinimide ester 1.3 0.8 [27]
Acetyl-Tryptophan methyl ester 3.9 2.2 [108]
Diethyl-2-amino-3-phenyl-propylphosphonate 2.3 ND [109]
Peptides
Phenylalanylglycine anilide 5.1 0.5 [26]
Cbz-Ala-Ala-OMe 3.2 4.5 TRIM [110]
Cbz-Ala-Gly-Phe-OMe 3.6 4.2 TRIM [110]
N-Ac-Phe-Trp-OMe 3.3 > 2 [31]
Cbz-Asp-Phe-OMe 2.5 VPY-MAA [111]
Commercial drugs
Propranolol 2.8 1.3 [112]
Timolol 2.9 2.0 [112]
Metoprolol 1.08 1.2 TRIM d [113]
Ephedrine 3.4 1.6 [29]
Naproxen 1.7 0.8 VPY [114]
Ropivacaine 7.7 / 5.7 f d [113]
Carboxylic acids
R-(–)-Mandelic acid 1.5 – VPy [108]
R-Phenylsuccinic acid 3.6 2.0 VPy [108]
2-Phenylpropionic acid high high PYAA/DVBd [96]
Amines
N-(3,5-dinitrobenzoyl)-methylbenzylamine 1.9 – MAA/DPGL [115]
(R)-α-methylbenzylamine >1.5 1.0 [116]
a ) Each racemate was applied on a polymer (ca. 0.1 µmol per gram dry polymer) imprinted with one antipode of
the racemate. The standard mobile phase, consisting of acetonitrile containing various amounts of acetic acid,
was used in most cases. Cbz = Carbobenzyloxy, Boc = t-butyloxycarbonyl.
b ) α was calculated as the ratio of the capacity factor (k’) of the template enantiomer to the capacity factor of its
antipode. R is the resolution factor.
s
c ) The polymers were prepared using MAA as functional monomer and EDMA as crosslinking monomer if not
otherwise noted. VPY= 2- or 4-vinylpyridine; TRIM = trimethylolpropane trimethacrylate; DPGL = (R)-N,O-
dimethacryloylphenylglycinol; PYAA = 3-(4-pyridinyl)acrylic acid.
d ) The polymer was evaluated in capillary electrophoresis.
e ) The polymer was imprinted with L-PA.
f ) Migration times of the two enantiomers.